A new class of anisimide-functionalized lipids that enables targeted delivery of lipid nanoparticles (LNPs) to activated fibroblasts in the liver.
Problem:
Lipid nanoparticles (LNPs) have been successfully used in FDA-approved therapies to deliver messenger RNA (mRNA) and small interfering RNA (siRNA) . One of the biggest challenge in developing a successful RNA therapies is its targeted delivery. Typically, LNPs require targeting ligands to be coated onto their surface to enable targeted delivery after the lipid is made. This added step often complicates the fabrication process.
Solution:
Rather than functionalizing LNPs with targeting ligands post-fabrication, this technology incorporates targeting capabilities directly into the lipid component to create ligand-thethered LNPs. This creates an affinity to the notoriously hard-to-target activated fibroblasts in the liver responsible for the buildup of collagen.
Technology:
The method incorporates small targeting moieties into the ionizable lipid which enable the generation of ligand-tethered LNPs. The lead AA-lipidoid AA-T3A-C12 mediates greater RNA delivery and transfection of activated fibroblasts than its analog without anisamide and the FDA-approved MC3 ionizable lipid. In a preclinical model of liver fibrosis, AA-T3A-C12 enables ~65% silencing of heat shock protein 47, a therapeutic target primarily expressed by activated fibroblasts, which is 2-fold more potent than MC3, leading to significantly reduced collagen deposition and liver fibrosis.
Advantages:
- Enables targeted LNP creation without the need for post-fabrication processes
- Can be used to deliver genetic medicines such as mRNA and siRNA therapies
- Increased selectivity and ability to target liver fibroblasts
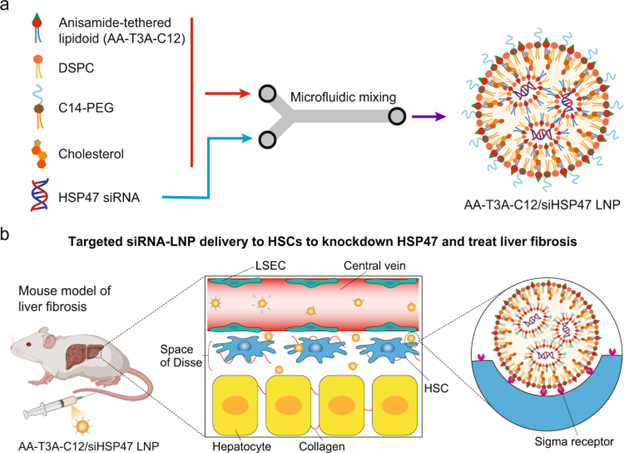
(a) Formulation of AA-T3A-C12/siHSP47 LNP via microfluidic mixing. (b) Scheme of targeted AA-T3A-C12/siHSP47 LNP delivery to activated HSCs to knockdown HSP47 and treat liver fibrosis. HSCs are located in the space of Disse, an area between LSECs and hepatocytes. After rapidly shedding PEG in circulation, the LNP exposes multivalent anisamide ligands on its surface that can strongly bind with sigma receptors overexpressed on activated HSCs to mediate cellular uptake. b was created with BioRender.com. Figure obtained from the inventor's Nature Communications publication.
Case ID:
22-9782-tpNCS
Web Published:
5/12/2023
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |