Compounds are designed to detect and characterize specific RNAs in a cell or sub-cellular compartment using light activation.
Problem:
Cellular RNAs provide critical information on gene expression and regulation. A variety of methods have been developed to isolate mRNA from target cells to understand gene function; however, these methods lack the ability to effectively detect and compare nucleic acid molecules longitudinally, or among different cells or subcellular compartments. Further, the ability to effectively capture all RNA, not just the mRNA transcripts responsible for protein translation, remains elusive. There is a need for tunable compounds that can be multiplexed to detect the nucleic acid fingerprint of single cells in live and fixed tissue samples in diverse biological contexts.
Solution:
A “caged” molecule that is activated with near-UV or visible light with single-cell and high spatiotemporal resolution – “caged” molecules are those whose function is blocked until activated by specific stimuli, allowing for tunable activation. The molecule can capture and label RNA of interest in live and fixed tissue for longitudinal transcriptomic detection over time.
Technology:
Light is often employed as an external stimulus to control biological processes. “Caged” biomolecules that are blocked until activated by specific stimuli are useful for this application. These molecules activate with near-UV or visible light, which introduce few side reactions and allow for convenient modulation with high spatiotemporal resolution. The “caged” molecules described here, transcriptome in vivo analysis (TIVA) and transcriptome in situ analysis (TISA)-tags, penetrate cells to isolate and label select RNAs through antisense oligonucleotide and ruthenium-based photolinker domains in a targeted manner.
Advantages:
- “Caged” tags that can be activated with near-UV or visible light, introducing few aberrant reactions, and allowing for high-resolution modulation.
- Stimuli applied and removed noninvasively and directed at a precise time to a specific location with variable intensity, offering fine-tuning.
- The light stimulus does not interfere with most natural biological processes.
- The tags can be designed for multiplexed detection of various RNAs of interest or longitudinal detection of RNAs over time.
- The molecular tags offer single-cell resolution and can be used in fixed or live tissue, broadening their use and application.
- The molecular tags can capture and label microRNAs, lincRNAs, intron-containing RNAs, hnRNAS, in addition to poly-A+ mRNA and polyA-mRNA.
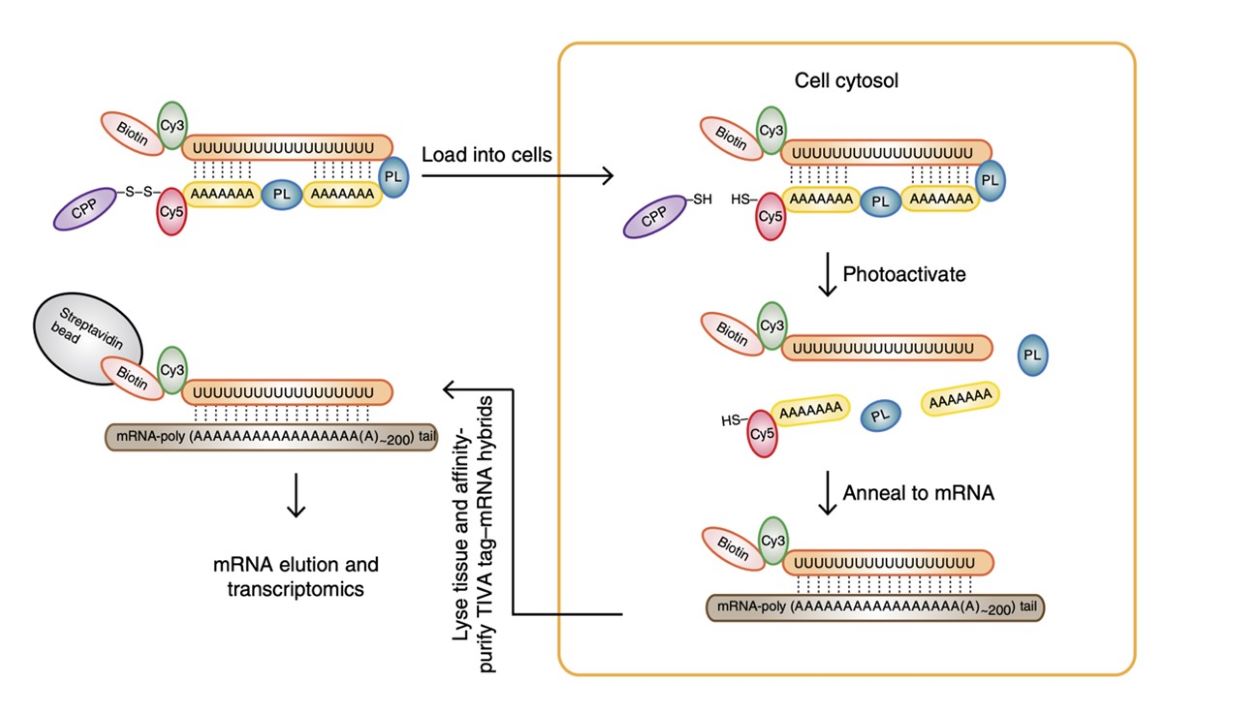
Schematic of the TIVA tag – a multifunctional, caged RNA-capture molecule. The TIVA tag is composed of several functional groups that facilitate cell entry, photoactivation, annealing to target mRNA and transcriptomic analysis. Figure located in Lovatt et al.,
Nat. Methods, 2014, 11 – 190 Figure 1, page 190.
Stage of Technology
- Proof of Concept
- Bench Prototype
Case ID:
15-7214-tpNCS-02
Web Published:
5/18/2023
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |