Lipid nanoparticles for selective mRNA delivery to the placenta to treat pre-eclampsia, FGR, and other pregnancy-related disorders.
Problem:
3-8% of pregnant women experience pre-eclampsia, or insufficient blood flow to the placenta. In severe cases, this can cause fetal growth restriction (FGR), in which the developing fetus cannot receive adequate blood flow. The only curative option for pre-eclampsia and FGR is to deliver the fetus regardless of gestational age. Because of this, pre-eclampsia and FGR are the leading causes of premature and stillbirths.
Several groups have developed gene therapies to try to address pre-eclampsia and FGR. However, these viral-based approaches have the potential for permanent off-target effects in other organs and must be delivered directly into the placenta via an invasive uterine injection.
Solution:
The inventors identified a lipid nanoparticle composition to deliver mRNA-based therapeutics to the placenta effectively. mRNA-based therapies are safer than viral alternatives as they last for a shorter time inside the body. The mRNA lipid nanoparticles can be loaded with mRNAs to treat pre-eclampsia, FGR, or other pregnancy-related disorders. They can be delivered through a regular IV without requiring invasive actions.
Technology:
The inventors designed and screened a library of 15 lipid nanoparticle compositions for their ability to deliver mRNA to placental cells. They then tested the lead compositions for their ability to target the uterus and placenta in mice. The lead LNP (A4) showed enhanced delivery to the placenta compared to an industry-standard LNP. Importantly, the LNPs delivered mRNA to the placenta, but not the fetuses, suggesting this therapy is safe for placental targeting.
The inventors then delivered VEGF mRNA, a vascular growth factor known to stimulate blood flow, via LNPs. The A4 LNP increased fetal blood vessel dilation, indicating on-target VEGF delivery.
Advantages:
- LNPs are delivered by IV, which is less invasive than the intrauterine injections used to deliver viral therapies
- mRNA-based therapies have fewer long-term risks than viral-based therapies
- The A4 LNP delivers more mRNA (62%) to the placenta than the industry standard LNP (34%)
- The A4 LNP delivers mRNA to the placenta but not the fetuses, suggesting fetal safety
- The A4 LNP delivers mRNA to more of the relevant cells (3.1%) than the industry standard LNP (1.7%)
- The A4 LNP shows a lower potential for liver toxicity than the industry standard LNP. (The industry standard shows 8.7-fold higher expression of a marker of liver toxicity than A4)
- LNP delivery shows no signs of causing placental inflammation
- The A4 LNP specifically increases fetal blood vessel dilation, while the industry standard LNP increases maternal blood vessel dilation. Fetal blood vessel dilation indicates increased on-target local delivery by A4, and maternal blood vessel dilation indicates broader systemic delivery by the industry standard
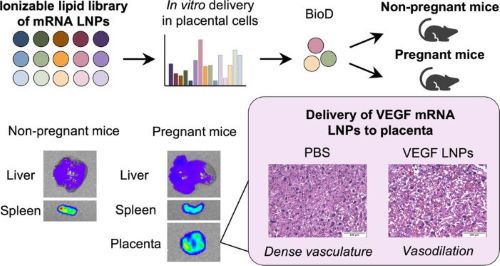
Engineered ionizable LNPs for mRNA delivery to the placenta with applications in mediating placental vasodilation.
Case ID:
22-10089-tpNCS
Web Published:
5/22/2023
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |