Problem:
Recording neuronal activity in vivo using optical methods can help to distinguish between normal and diseased brains. An ideal electrophysiological sensor would have a customizable structure and mechanism, providing excellent spatial contrast while minimizing background noise. However, current genetically encoded protein-based voltage indicators (GEVIs) face two significant limitations. The first is slow temporal resolution due to structural reorganization during voltage sensing. The second is engineering constraints imposed on natural proteins, as protein engineering can lead to dramatic changes that compromise proper folding and the function of protein machines.
Solution:
To overcome the limitations of current GEVIs, researchers have proposed a new approach for creating next-generation sensors through rational design using non-biologically derived proteins from first principles. This approach is not limited by the constraints of modifying natural proteins, enabling the optimization of molecular content and biophysical mechanisms to achieve outstanding temporal resolution, sensitivity, and adaptability.
Technology:
The inventors used maquette technology to design GEVIs. Maquettes are rigid 4-helix bundle proteins that act as custom scaffolds for biological co-factors like fluorophores or redox sensors. During voltage sensing, the fluorophore within maquette GEVIs provides an ultrafast fluorescence signal change independent of structural reorientation. The physical-chemical properties of GEVIs can be easily tuned by re-engineering the maquette without the risk of protein unfolding, as the starting maquette is stable and robust. Furthermore, this technology can be applied to other physiological reporters by changing the combination of cofactors anchored to the maquette.
Advantages:
- High tolerance to sequence modification, redesign, and tuning of maquette functional properties
- A biosensing platform for different electrophysiological signals
- Ultrafast response with outstanding temporal resolution and sensitivity
- Expressable in mammalian cells
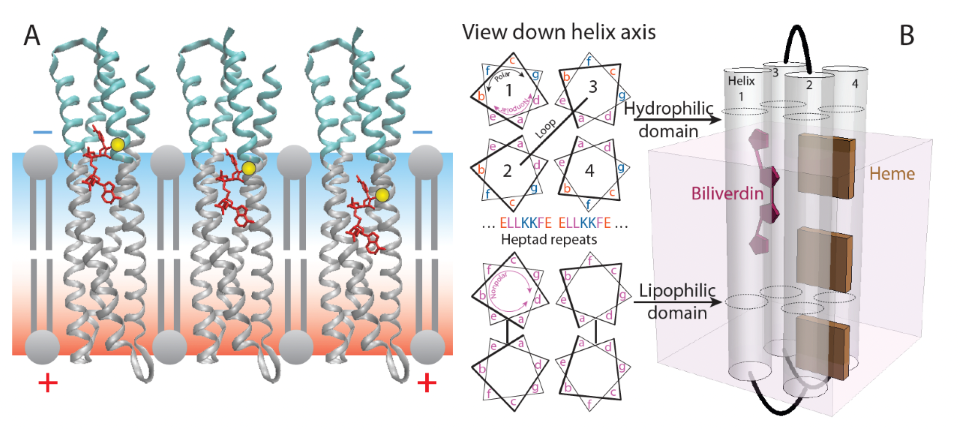
(A) Transmembrane 4-helix bundle maquette with polar (cyan) and nonpolar regions (gray) using cysteine (yellow) to anchor a field-sensing biliverdin fluorophore (red). (B) Sequences are based on heptad repeats that place polar groups on one face of the helix and nonpolar groups in membrane-spanning regions.
Case ID:
15-7570-tpNCS
Web Published:
6/7/2023
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |