Small molecule therapeutics that aid in clearing abnormal protein deposits observed in neurodegenerative diseases.
Problem:
Dementia, characterized by the impairment of at least two brain functions, such as memory loss and judgment, currently has no cure. Two common neurodegenerative diseases that cause dementia are Frontotemporal Dementia (FTD) and Alzheimer’s Disease (AD). In the United States, FTD affects an estimated 50,000 to 60,000 people, and AD, more than 6 million people. These diseases are characterized by the abnormal accumulation of protein aggregates, specifically tau and TDP-43 aggregates. Tau and TDP-43 proteins undergo abnormal folding and clump together into aggregates, which disrupt normal cellular function. Valosin-containing protein (VCP) is responsible for solubilizing and breaking down the tau and TDP-43 aggregates, preventing their accumulation, but pathogenic variants of VCP impair the breakdown of protein aggregates. Notably, other neurodegenerative diseases, such as Paget’s disease of bone, Parkinson’s Disease, and amyotrophic lateral sclerosis, are also associated with pathogenic variants of VCP.
Solution:
The inventors identified four small molecules that allow pathogenic VCP to effectively unfold and degrade the insoluble aggregated forms of tau and TDP-43.
Technology:
VCP plays a role in protein quality control, and many ubiquitinated proteins (proteins tagged by ubiquitin for degradation) require pre-processing by VCP to be degraded by the proteasome. From a panel of approximately one hundred structurally distinct small molecules, the inventors discovered four compounds that increase VCP’s ATPase activity, allowing VCP to remove protein aggregates more efficiently.
Advantages:
- VCP activity decreased tau filament aggregation by 30.1%.
- Small molecules increased VCP ATPase activity by more than 2 standard deviations from the mean and showed better clearing of protein aggregates.
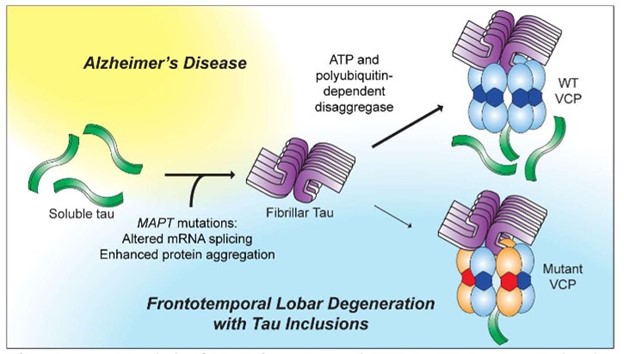
A schematic highlighting mechanisms that lead to neurodegeneration in Alzheimer’s Disease and Frontotemporal Lobar Degeneration. Soluble tau proteins aggregate to form fibrillar structures leading to disease. At the top right, wild-type VCP solubilizes and breaks down the problematic fibrillar tau. Mutant (pathogenic) VCP, on the other hand, impedes this process (lower right). The four discovered VCP compounds overcome the effects of pathogenic VCP and increase the efficiency of wild-type VCP for clearing protein aggregates.
Case ID:
22-9828-TpNCS
Web Published:
9/15/2023
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |