A class of pyrazole derivatives that inhibit palmitoyl-transferases, resulting in increasing cancer cell responsiveness to epidermal growth factor receptor inhibitor and KRas mutant lung cancer growth inhibition as a single agent.
Problem:
Pancreatic and lung cancers are largely driven by either Epidermal growth factor receptor (EGFR) mutation KRAS mutations. However current therapies targeting either of these mutated proteins are either unavailable (KRas G12S/D/V/R) or rapidly develop resistance mutations (EGFR, KRas G12C). No targeted therapeutic options exist for mutant KRAS G12S/D/V/R tumors, making treatment of these cancers difficult and dependent on conventional chemotherapy and radiation.
Solution:
Pyrazole derivatives that increase sensitivity to EGFR inhibitors by creating a dependency on EGFR signaling for cancer cell survival, increasing responsiveness to EGFR inhibitor therapy. Additionally, the increase in EGFR signaling induces synthetic lethality in KRAS-mutation-bearing tumors. This treatment circumvents acquired resistance to EGFR inhibitors and offers a therapeutic strategy for mutant KRAS tumors.
Technology:
The pyrazole derivatives target an EGFR palmitoylation enzyme, DHHC20, which modulates EGFR signaling. When DHHC20 is inhibited, EGFR signaling becomes crucial for cancer cell survival. Inhibiting DHHC20 also causes synthetic lethality in mutant KRAS tumors because oncogenic mutations in KRAS and EGFR are mutually exclusive, and the co-occurrence of these mutations is deleterious to the cancer cell.
Advantages:
- A first-in-class drug that increases the response to current EGFR inhibitor drugs, increasing efficacy for these treatments in lung cancer patients.
- Offers a therapy independent of the type of KRAS mutation on the tumor, allowing for reliable treatment of these types of tumors via a single agent.
- Target pyrazole derivatives have been shown to inhibit KRAS mutant lung tumor growth in xenografts, significantly increasing overall survival with no impact on animal weight following oral dosing, demonstrating therapeutic potential.
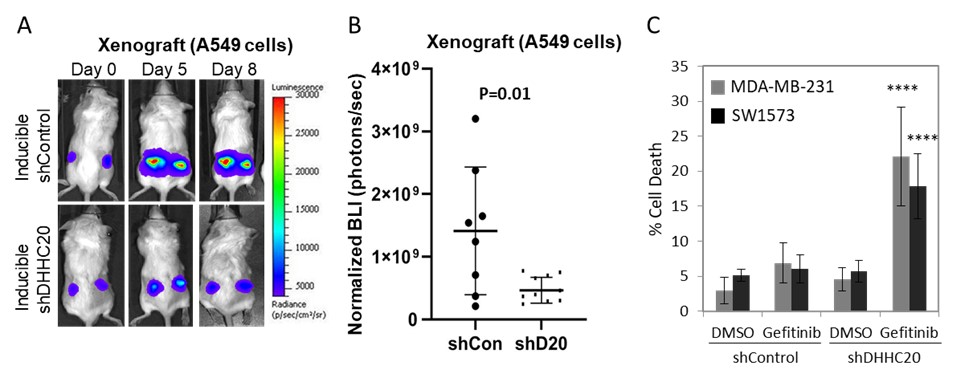
(A) Imaging of xenograft tumors grown from A549 (Kras G12S) human lung cancer cells stably transduced with inducible control shRNA or DHHC20 shRNA. shDHHC20. (B) Quantification of tumors on day 8 after shRNA induction with tamoxifen. (B) Silencing DHHC20 expression by shRNA increases sensitivity to 5 μM gefitinib (EGFR inhibitor) induced cell death in KRAS mutant breast (MDA-MB-231) and lung (SW1573) cancer cells.
Stage of Development:
- Target Identified
- Preclinical Discovery
- Preclinical in vivo Proof of Concept with oral dosing
Reference Media:
- Kadry, YA et al. Open Biol 2021 Oct, 11(10): 210033.
- Nelson, JC et al. Curr Biol 2020 July, 30(14): 2729.
- Kharbanda, A et al. Sci. Signal., 2020 March 3, 13(621): eaax2364.
- Kharbanda, A et al. Biochem Biophys Res Commun 2017 Nov 4, 493(1): 213.
- Runkle,KB et al. Mol Cell 2016 May 5, 62(3): 385.
Case ID:
23-10336-TpNCS
Web Published:
11/20/2023
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |