Viral vector-mediated gene silencing of a novel short retinal isoform of Prolactin promotes photoreceptor survival.
Problem:
Age-related macular degeneration (AMD) and inherited retinal degeneration (IRDs) have extreme mutational heterogeneity, and many IRDs are orphan diseases. Developing individual gene therapies for each causative gene is impractical and cost-ineffective. Therefore, developing universal therapeutic options that can prolong photoreceptor survival for various forms of retinal degeneration remains the holy grail for treating IRDs and AMD.
Solution:
The inventors have identified a novel isoform of full-length prolactin (PRL) lacking the first exon, PRLΔE1, expressed explicitly in diseased rod photoreceptors. An shRNA-mediated gene therapy designed to knockdownPRLΔE1 demonstrates an initial protective effect in the retina, supporting PRLΔE1 knockdown as a candidate strategy for a “gene agnostic” therapy for retinal degenerations.
Technology:
It is established that the expression of the PRLΔE1 isoform is strictly correlated with photoreceptor disease. Subsequently, the shRNA-mediated knockdown of PRLΔE1 was tested in two models of retinal degeneration that express PRLΔE1, PDE6β-RCD1, and RPGR-XLPRA2. The shRNA targeting PRLΔE1 is packaged in an AAV2/5 vector for sustained shRNA expression and delivered subretinally at a predetermined optimal titer. As a result, PRLΔE1 knockdown is found to preserve the thickness of the outer nuclear layer (ONL), the retinal cellular layer harboring the photoreceptor cell bodies.
Advantages:
- Four new PRLΔE1-targeting shRNAs are identified after stringent in silico screening for potential off-target effects.
- Retinas treated with the shRNAPRLΔE1 preserve the outer nuclear layer at five weeks post- injection.
- The protective effect of silencing PRLΔE1 expression is confirmed in two non-allelic models of inherited retinal degeneration.
- A potential “gene-agnostic” therapy for retinal degeneration.
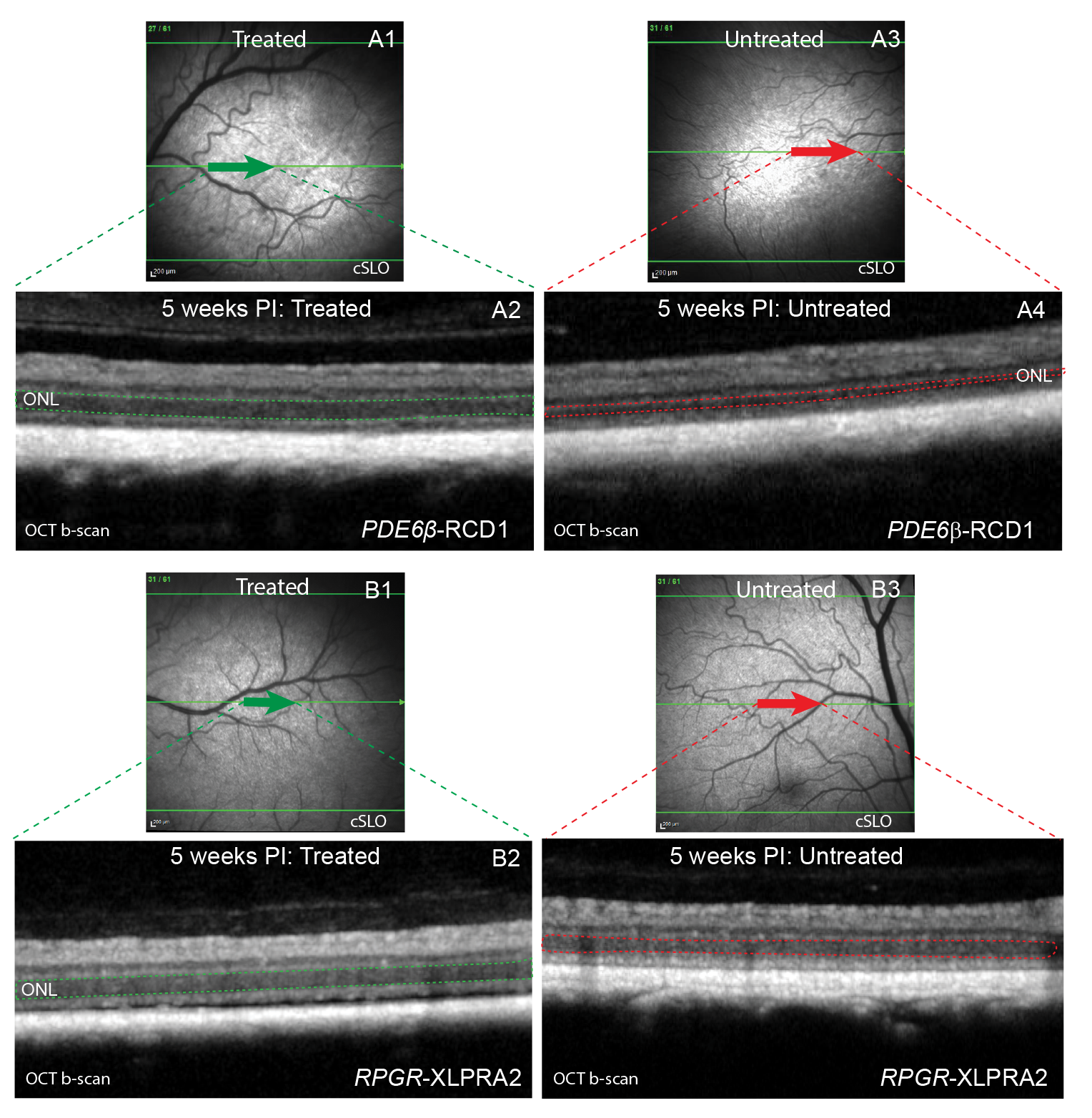
In vivo assessment shows preserved ONL thickness 5 weeks after treatment with shRNAPRLΔE1 in PDE6β-RCD1 (A) and RPGR-XLPRA2 (B) retinas. cSLO (A1 and B1) and OCT b-scan (A2 and B2) images showing treated retinal area; cSLO (A3 and B3) and OCT b-scan (A4 and B4) images of the untreated retinal area. The ONL is highlighted in the OCT b-scan images with dotted lines within the treated (green) and the untreated (red) retina in PDE6β-RCD1 (A2, A4) and RPGR-XLPRA2 (B2, B4).
Intellectual Property:
- US Provisional Application Filed
Case ID:
24-10520-TpNCS
Web Published:
3/12/2024
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |