Enzymes that degrade mannose to prevent the formation of bacterial-fungal biofilms, which cause dental cavities
Problem:
Dental cavities are among the most common oral diseases among children and adults in the United States, with treatments for this condition exceeding $81 billion annually. Dental cavities occur when the bacteria Streptococcus mutans feeds off residual sugar on teeth and produces tooth-decaying lactic acid. Additionally, S. mutans can cause even more severe tooth decay when it associates with the fungus Candida albicans. A surface-bound carbohydrate, mannose mediates synergistic growth of bacteria/fungus colonies by helping the two species bind to each other. Disruption of mannose-mediated bacterial-fungal biofilms interaction on teeth would prevent severe dental cavities caused by these two pathogenic microorganisms.
Solution:
The technology is mannan-degrading enzymes (MDEs) which break down mannose on Candida albicans cell walls to prevent bacterial binding. Specific degradation of mannans on fungal cell walls stops Streptococcus mutans from adhering to the fungal cells—consequently, less co-species biofilm forms on teeth. Limiting biofilm formation decreases the exposure of teeth to microbe-generated acids and inhibits the formation of dental cavities.
Technology Overview:
Three mannan-degrading enzymes are used to target fungal cell walls. The enzymes: α-mannosidase, β-mannosidase, or β-mannanase breakdown mannose on Candida albicans surfaces. These enzymes target beta-mannose sugars on mannan chains. Ultimately, this reduces the viscosity of biofilms and removes binding sites used by Streptococcus mutans on enamel.
Advantages:
- 15-fold decrease in S. mutans-C. albicans interaction force after 5-minute treatment
- 2.5-fold decrease in biofilm dry weight
- Up to 90% biofilm removal by mild fluid shear stress after treatment
- Increases pH on mineral surfaces (less acidity) and decrease in enamel demineralization
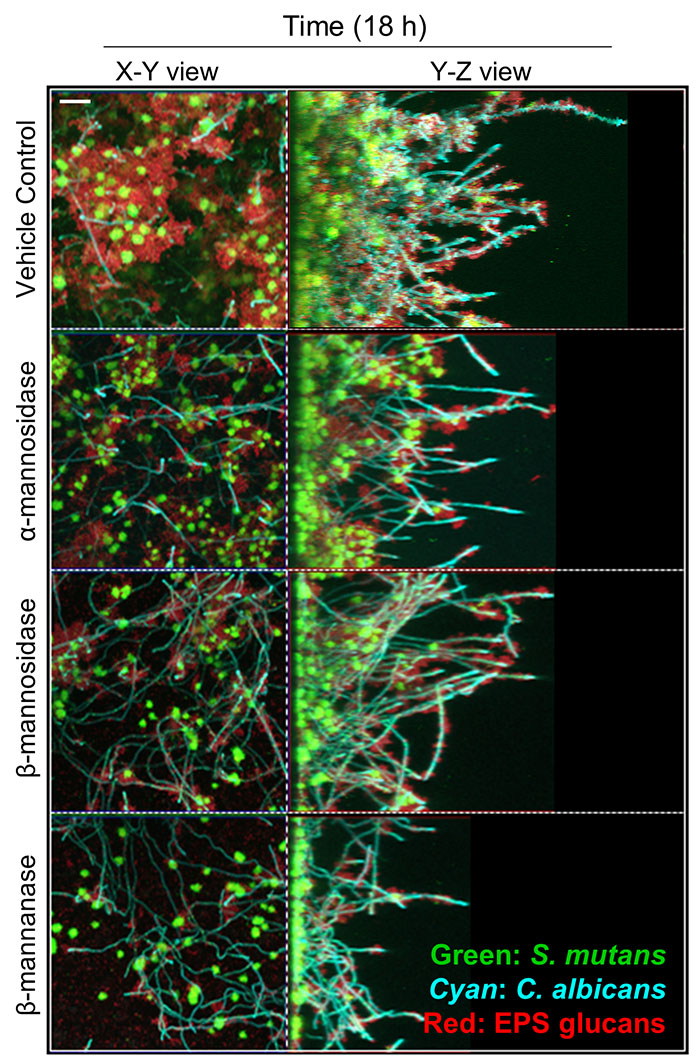
Efficacy of MDEs against S. mutans-C. albicans biofilms. (A) the pH of biofilm supernatants, (B) dry-weight per biofilm, CFU of (C) S. mutans, and (D) C. albicans per biofilm. At optimal units, all MDEs had a significant antibiofilm effect on S. mutans-C. albicans biofilms as measured at 18, 28, and 42h. (E) Representative confocal images of untreated and MDE treated 746 biofilms at 18 h. Scale bar indicates 20 µm. Statistics: *** represents p < 0.001 for unpaired t-tests against the vehicle control (n≥3).
Stage of Development:
- Therapeutics: Preclinical Discovery
Case ID:
21-9552-tpNCS
Web Published:
4/6/2021
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |