Novel high-affinity PARP inhibitors with various properties for imagining and treating cancer and inflammation-related diseases
Technology Overview:
The poly(ADP-ribose) polymersase-1 (PARP-1) enzyme is heavily involved in propagating neurodegeneration and cancer. In neurodegeneration PARP-1 causes cell death, while in cancer PARP-1 acts as DNA damage response enzyme that promotes survival of cancer cells with mutations within DNA repair genes. Therefore, PARP-1 inhibitors are used for treatment of cancers that harbor DNA repair genes mutations, such as BRCA-1 and BRCA-2. PARP-1 can also serves as a target for delivery of therapeutics radionuclides including alpha, beta, and auger emitting radionuclides. In neurodegeneration, PARP-1 can serve as a biomarker of neurodegeneration and as a therapeutic target for treating neuroinflammation.
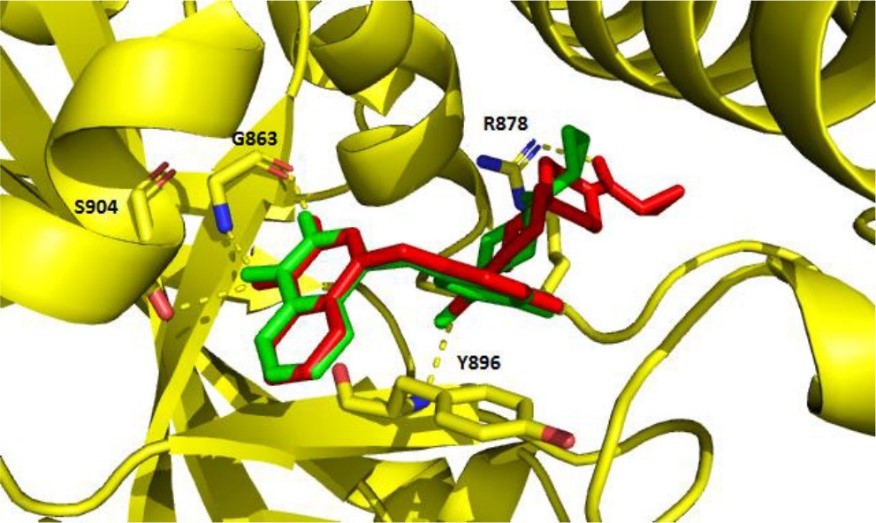
Dr. Mach and his team at Penn investigated the pharmacological impact of incorporating spirodiamine motifs into the phthalazine architecture of FDA approved PARPi olaparib. The team developed several first in class compounds that show high PARP-1 affinity and various catalytic inhibition, DNA damage properties, and cytotoxicity. These new drugs potentially can be used
- as PET imaging agents for neurodegenerative diseases;
- as PET diagnostic agents for determining PARP-1 expression in patient’s tumor prior to treatment with PARP-1 inhibitors;
- to deliver cytotoxic radionuclides directly to cancer chromatin;
- for treatment of PARP-1-mediated neurodegeneration;
- for treatment of cancers harboring DNA repair genes mutations; and
- for treating inflammatory related diseases.
Advantages:
- High PARP-1 affinity
- Various cytotoxicity
- Reduced drug efflux through pump P glycoprotein
- Potentially increased access to brain
Applications:
- Cancer imagining, therapy, and radiotherapy
- Neuroimaging and neuroinflammation treatment
Stage of Development:
- In vitro data
- Ongoing in vivo experiments
Reference Media:
- Lengyel-Zhand, Z et al.; Pharmacol Ther 2022 Feb 230: 107968.
- Puentes, LN et al.; Mol Neurobiol 2021 Aug 58(8): 3641 (pdf)
- Puentes, LN et al.; Front Aging Neurosci 2021 Jun 18 13: 704041
- Reilly, SW et al. J Med Chem, 2018 Jun 28, 61(12): 5367
- Reilly, SW et al. ACS Omega, 2018 Aug 31, 3(8): 9997
- Reilly, SW et al. Bioorg Chem, 2019 March, 83: 242
Case ID:
18-8465-tpNCS
Web Published:
2/16/2022
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |