Peptides derived from the human apolipoprotein B that are antimicrobial, anti-inflammatory, highly cytotoxic, and do not select for bacterial resistance.
Problem:
In 2019, more than 2.8 million people in the U.S. suffered from antibiotic-resistant infections, leading to over 35,000 deaths. Such infections are of great concern, especially for ESKAPE pathogens, which have growing resistance to commonly used antibiotics. It has been estimated that 10 million people would die each year from these untreatable diseases by the year 2050 at the present rate. Therefore, there is an urgent need to develop alternatives to conventional antibiotics to treat drug-resistant infections.
Solution:
Four encrypted peptides that have been derived from human apolipoprotein B exhibit antimicrobial, anti-inflammatory, and highly cytotoxic properties. When combined, the peptides potentiated the activity of conventional antibiotics against bacteria and do not select for bacterial resistance. In addition, a retro-inverso variant of the lead encrypted peptide that is protease-resistant was produced and showed anti-infective efficacy in a mouse model.
Technology:
The inventors identified a class of encrypted peptides derived from the plasma protein human apolipoprotein B by using an algorithm developed by their group. Three ApoB-derived peptides were demonstrated to exhibit potent antimicrobial activity against drug-resistant Klebsiella pneumoniae, Acinetobacter baumannii, and Staphylococci both in vitro and in an animal model. In addition, a protease-resistant variant was synthesized by reversing its peptide sequence, to avoid proteolytic degradation and ensure efficacy against common hospital-acquired infections.
Advantages:
- The peptides were found to exert significant antibacterial effects (minimal inhibitory concentration ranging from 2.5−20 µmol L-1) against 5 key pathogenic genera implicated in hospital-acquired infections including Staphylococcus, Acinetobacter, Klebsiella, Enterococcus, and Pseudomonas.
- (ri)-r(P)ApoBsPro has increased peptide stability as its antimicrobial activity remained constant after 16 h of preincubation in serum, while its parent peptide r(P)ApoBsPro completely lost its activity upon incubation.
- (ri)-r(P)ApoBsPro increases the translational potential of its parent peptide without altering its antimicrobial and cytotoxic activities.
- The strategy of synthesizing a retro-inverso variant makes the molecules more suitable for future preclinical and clinical studies.
- When combining ApoB-derived encrypted peptides with conventional antibiotics, synergistic effects were detected and the peptides potentiated the activity of conventional antibiotics against bacteria and do not select for bacterial resistance.
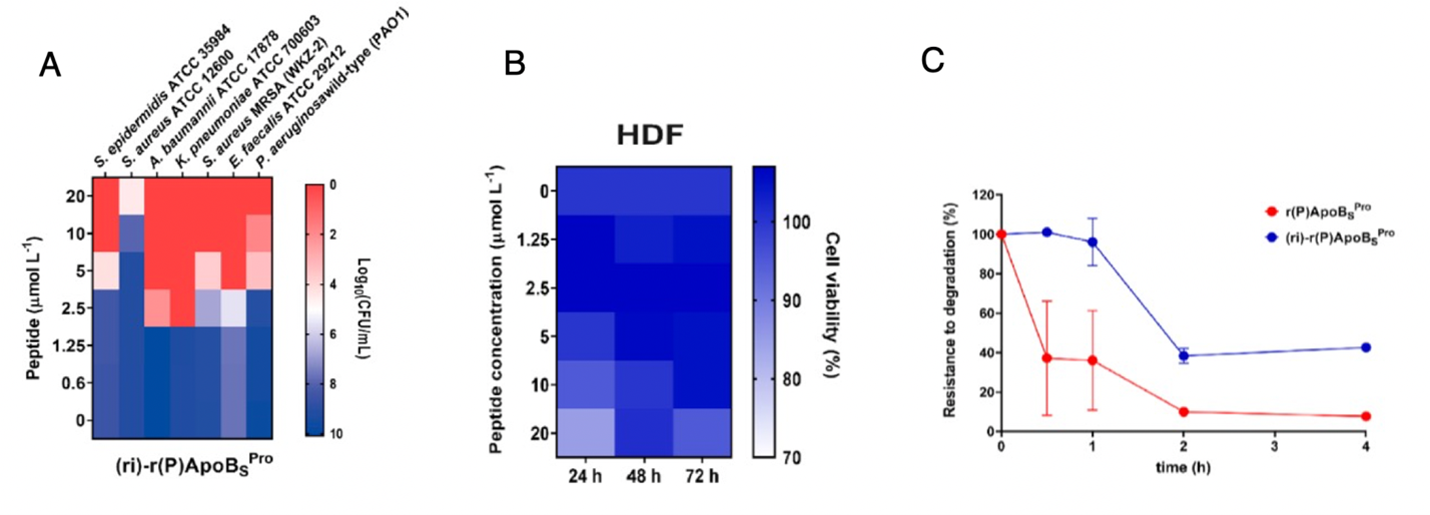
Figure caption: (A) Antimicrobial activity of (ri)-r(P)ApoBsPro (µmol L-1) against seven bacterial strains. (B) Cytotoxic effects of increasing concentrations of retro-inverso ApoB ((ri)-r(P)ApoBsPro) on HDF (human dermal fibroblasts) cells. (C) Resistance to degradation of (ri)-r(P)ApoBsPro exposed to fetal bovine serum (FBS) proteases for 4 h.
Intellectual Property:
- US Utility Patent Application Filed
Case ID:
22-9974-tpNCS
Web Published:
6/15/2023
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |