Synthetic antimicrobial peptides derived from wasp venom to treat antibiotic-resistant infections.
Problem:
Antibiotics have transformed modern medicine as they are just one of the most effective drugs in history. However, the rise of antimicrobial resistance (AMR) currently threatens to undermine these achievements, posing a major threat to public health. Millions of patients worldwide, including tens of thousands in the US, die each year from untreatable infections due to the emergence and spread of antibiotic resistance while the arsenal of effective drugs shrinks. As a result, the market for these drugs is set to grow 3-7% until 2030. Venoms represent an underexplored potential source of new antibiotics; however, they are limited by their toxicity.
Solution:
Here, rationally designed, synthetic variants of two natural or wild-type (WT) antimicrobial peptides (AMPs) found in Eumenes micado wasp venom are non-toxic against human cell lines, active in an in vivo mouse model, have enhanced antimicrobial activity against Gram-negative bacteria, and a reduced propensity toward antibiotic resistance.
Technology:
Naturally occurring peptides found in Eumenes micado wasp venom have antimicrobial properties. Through structure-function-guided design including alanine and lysine screening, the inventors developed synthetic variants of two WT AMPs from the Eumenes micado wasp venom. Synthetic peptides with improved physicochemical properties such as net charge, helicity and amphiphilicity, were then subjected to in vitro toxicity and antibiotic efficacy and resistance studies compared to the WT peptides and conventional antibiotics, such as ciprofloxacin and polymyxin B. Those synthetic peptides that were not selected for antibacterial resistance and had improved antimicrobial activity were also non-toxic and had potent bactericidal activity in a preclinical mouse model, which confirm their improved performance in vivo.
Advantages:
- Reduced in vitro aggregation and increased amphiphilicity compared to the WT peptides, both of which are beneficial for manufacturing purposes
- No development of antibiotic resistance in Escherichia coli over the course of a 20-day treatment compared to ciprofloxacin, whose minimal inhibitory concentration (MIC) increased 1,000-fold over the same time course
- Non-toxic at concentrations 4- to 8-fold above their therapeutic dose in primary human keratinocytes and no side effects or toxicity in vivo (indicated by no momentous change in the body weight of the treated mice compared to untreated mice)
- A 38- to 4,600-fold reduction in Pseudomonas aeruginosa ATCC strain PAO1 bacterial titer in mouse model of infection
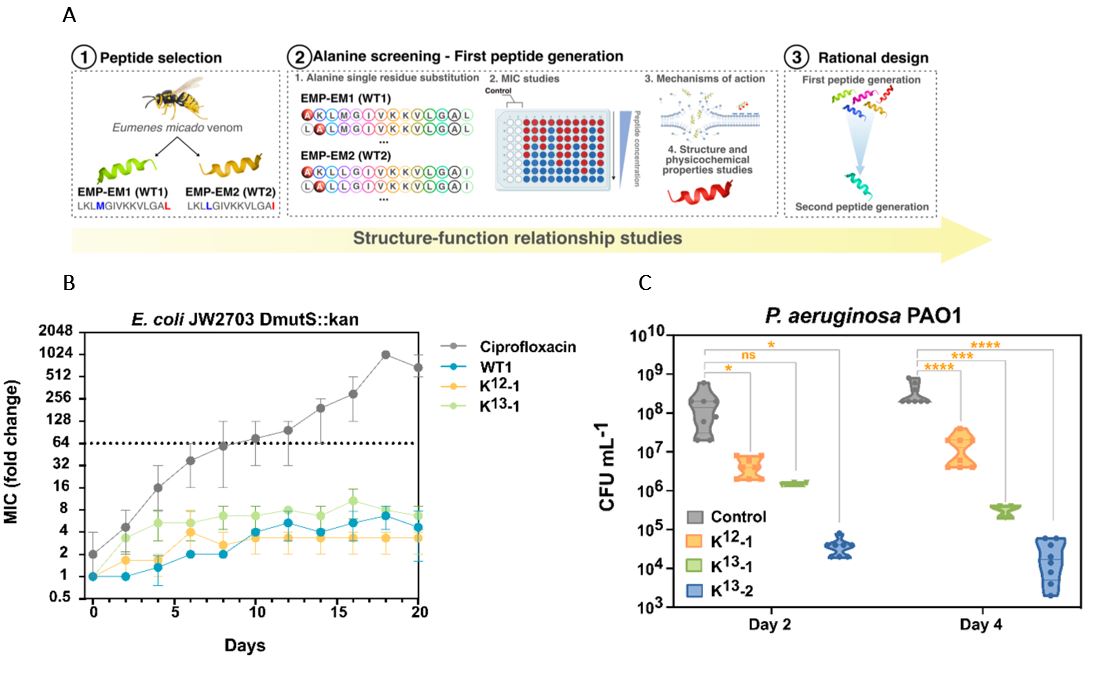
(A) A schematic describing the structure-function study design leading to second generation, synthetic peptides. (B) Antibiotic resistance studies of two lead synthetic peptides, K13-2, and K12-1, compared to one WT peptide and a conventional fluoroquinolone antibiotic, ciprofloxacin, utilizing the bacterium E. coli JW2703 DmutS::kan hypermutant strain from the Keio collection, and measured by minimum inhibitory concentration (MIC) over a 20-day time course. AMPs did not produce AMR whereas ciprofloxacin produced AMR after four days. (C) Results of an in vivo mouse model of infection whereby mice infected via an incision in the back with P. aeruginosa ATCC strain PA01, treated second-generation peptides, K12-1, K13-1, or K13-2, versus a WT control 1h post-infection. Quantification of bacterial load (by colony forming unit (CFU) per milliliter (mL)) from skin tissue samples collected 5 days post-infection showed that synthetic AMPs have improved antimicrobial activity compared to WT.
Stage of Development:
- Target Identified
- Preclinical Discovery