An ultrasensitive diagnostic tool that isolates single extracellular vesicles (EVs) and measures their contents to single molecule resolution.
Problem:
EVs contain biomolecules that can identify a variety of disease states and, thus, have emerged as a promising source for diagnostics. Within the EV diagnostic field, there are two major challenges: (1) the isolation and compartmentalization of single EVs to resolve their heterogeneity and (2) the precise measurement of the proteins contained within them, which tend to be of very low abundance. Previous attempts to isolate and analyze the contents of single EVs rely on bulk analysis of their molecular cargo and include techniques like modified flow cytometry and fluorescence imaging. However, these technologies have limitations of the inability to accurately quantify EV molecular cargo with ultra-high resolution.
Solution:
Here, a double digital assay overcomes the challenges of isolating and compartmentalizing single EVs as well as detecting their low-abundance proteinaceous contents. This technology flows a sample containing EVs over a microwell array and relies on the Poisson distribution to load single EVs into a single microwell. Then, an ultrasensitive digital enzyme-linked immunosorbent assay (dELISA) detects target proteins to single molecule resolution.
Technology:
This technology comprises two layers: a flow cell (top layer) and a honeycomb-inspired microwell array (bottom layer). Antibody-conjugated beads and spacer beads coat the bottom of the microwells. Sample containing EVs is flowed over the microwells, loading single EVs into single microwells. This technique relies on the Poisson distribution (l=0.1) to isolate single EVs whereby the statistical likelihood of two EVs being deposited into one well is very low. The EVs are then lysed, and their proteinaceous contents detected by dELISA, using tyramide signal amplification (TSA), which can detect analytes to single molecule resolution.
Advantages:
- Does not require expensive equipment nor technically challenging methods of separation
- Allows for the detection of rare biomarkers that may be missed with bulk analysis techniques
- Allows for detection of low abundance biomarkers that may be important for early detection of cancer and other diseases
- Samples may be taken from patients’ blood, which is convenient and non-invasive compared to procedures such as tissue biopsy
- Significant (100-fold) improvement in the limit of detection over conventional ELISA
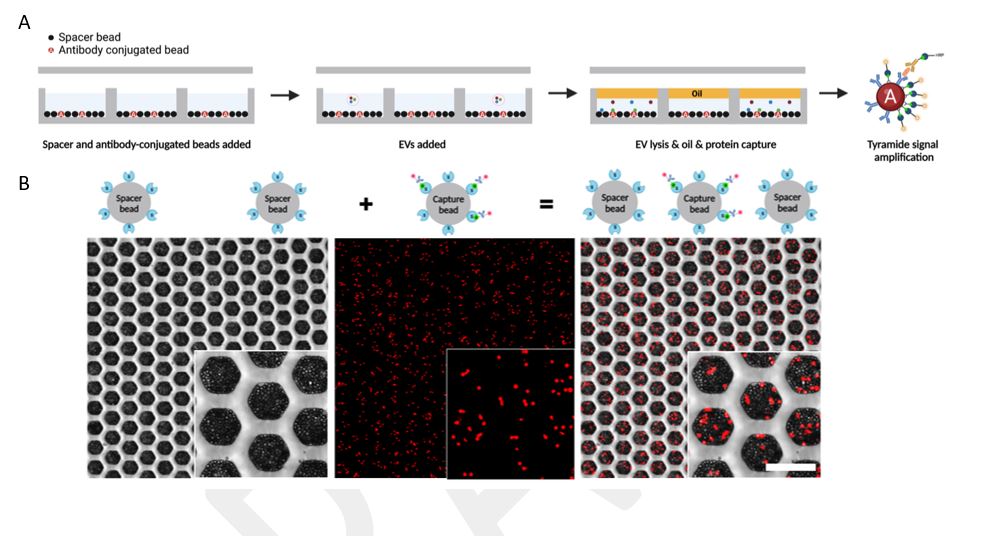
Figure 1. (A) Schematic depicting the experimental overview of the double digital EV assay. Microwells are coated with antibody-conjugated beads separated by spacer beads. Sample containing EVs is flowed over the microwells whereby single EVs are deposited into single wells. Lysis buffer is then flowed into the wells to release the contents of the EVs into the microwells followed immediately by oil to prevent cross-contamination. The contents of EVs are then captured by digital ELISA enhanced by TSA, which produces the fluorescent signal used to detect single protein molecules. (B) Honeycomb-inspired microwells demonstrating raw signal output of the digital ELISA enhanced by TSA from single EV samples.
Stage of Development:
- Proof of Concept
- Bench Prototype
Reference Media:
- Reynolds, D. E. et. al., Methods Mol Biol., 2023; 2689: 211
- Morales, R.-T. T., & Ko, J. et al., ACS Nano., 2022 Aug 23; 16(8): 11619
- Dr. Jina Ko, Schmidt Science Fellows, inSPIREd; 2019 July 26, Can a fusion of disciplines help us to fight rare diseases?
Case ID:
23-10430-tpNCS
Web Published:
10/9/2023
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |