Problem:
While the 5-year relative survival rate for patients with local or regional prostate cancer is nearly 100%, the 5-year relative survival rate drops to 32% for patients with metastatic or advanced disease. These patients may initially respond to available therapies, but most patients will develop therapy resistance and succumb to disease.
Solution:
A CAR construct that targets specific sugar-bearing prostate cancer cells and a subset of breast cancer cells.
Technology:
The inventors designed a CAR construct that targets metastatic prostate cancer, based on the first structurally defined F77 sugar antigen. About 85% of metastatic prostate cancer cells express this unique antigen. This novel CAR construct exhibited exquisite specificity and efficacy in highly relevant human prostate cancer cell lines, RWPE1 and RWPE2. F77 CAR-T cells effectively killed prostate cancer cells both in vitro and in vivo models.
Advantages:
- F77 CAR-T kills androgen-independent prostate cancer cells (these may include androgen negative cancer and hormone therapy resistant cancer)
- F77 expression is restricted to prostate cancer and a small subset of breast cancer– limiting off-target effects
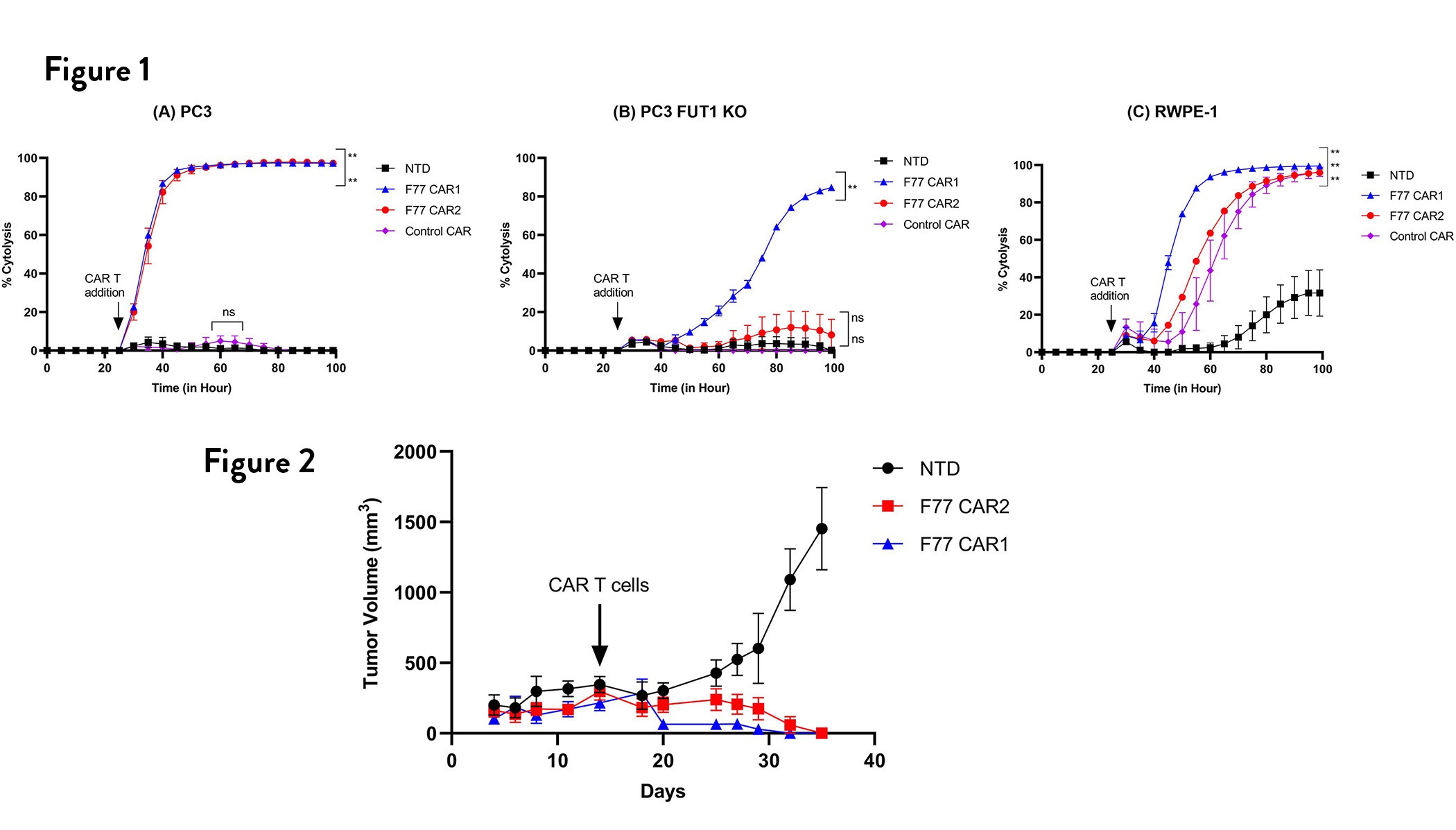
Figure 1. % Cytolysis determined through xCELLigence impedance measurement at 5:1 E:T ratio of CAR T cells and cancer cells. % Cytolysis for cancer cells was calculated after 24 h of cancer cell addition (A) PC3, (B) RWPE-1, (C) RWPE-2, and every 5 h after effector cells addition. Impedance monitoring was validated using T cells without any CAR (NTD), F77 CAR1, F77 CAR2 and control CAR effector (cells over GFP labeled target cells at E:T ratios of 5:1. Normalized Cell Index (NCI) was used to calculate % Cytolysis and plotted ± SEM. Significance of difference in activity of each CAR compared to NTD was determined using One-way ANOVA followed by Dunnett's correction for multiple comparisons. Asterisks indicate significance (** p<0.0001 and ns refers to p≥0.05).
Figure 2. Treatment of mice bearing PC3 subcutaneous tumors with F77 effector cells leads to tumor regression (n=5).
Stage of Development:
- Proof of concept in vitro and in vivo human prostate cancer xenograft mode
Case ID:
21-9684-TpNCS
Web Published:
10/25/2023
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |