Flow cytometry-based method for predicting post-surgical cardiovascular events.
Problem:
Cardiovascular events that occur after non-cardiac vascular surgery are significantly associated with post-surgical mortality. In fact, the mortality rate of patients who experience heart attacks following non-cardiac vascular surgery is 65%. Additionally, one in five patients suffer a cardiovascular event within one year of surgery. Stratification of patient population by risk factors is crucial to inform management of pre-, peri-, and post-surgical care for better outcome. Various intervention clinical trials aimed at improving surgical outcomes have produced conflicting results. A more precise and systematic approach is necessary.
Solution:
The inventors developed a flow cytometry-based method of analyzing extracellular vesicles (EVs) in patient samples collected prior to surgery to predict post-surgical cardiovascular event risk.
Technology:
The inventors demonstrated that surface markers on EVs collected prior to surgery (including CD31, CD105, and CD64) may predict risk of cardiovascular events following a non-cardiac surgical procedure (full panel includes: CD47, CD3, CD31, CD105, CD64, CD41a, and CD144). Analysis of EVs was done by 1) collecting pre-surgical patient blood samples, 2) isolating EVs, 3) labeling EV isolate, and 4) analyzing via high-sensitivity flow cytometry. The presence of EVs expressing CD31+, CD105+, and CD64+ was significantly correlated with cardiovascular events.
Advantages:
- Cost effective
- Rapid analysis
- Can achieve higher sensitivity and specificity in predicting cardiovascular events than any existing methodology.
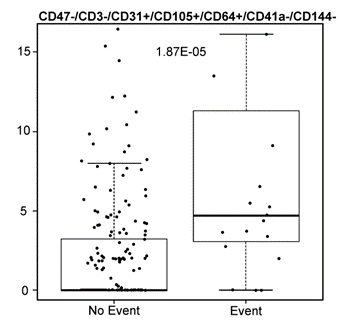
Graph comparing the discovered EV phenotype in the two groups of patients. Heavy line corresponds to the median value within groups. Box boundaries are the 1st and 3rd quantiles. Each dot corresponds to an individual patient. The y-axis has units of “EVs per ul Plasma”.
Stage of Development:
- Proof of concept demonstrated with patient samples in a prospective multi-site clinical trial (N=212).
Case ID:
18-8716-TpNCS
Web Published:
6/17/2024
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |