A rapid, low-cost, portable swab-based SARS-CoV-2 biosensor whose test result can be detected with naked eye
Problem:
COVID-19 has killed over 2.84 million people worldwide and has disproportionately affected people in lower-income and resource-limited geographies. High-frequency testing can prevent rapid virus spread and reduce COVID-19-related hospitalizations and deaths, but this can only be realized with accessible, low-cost, and rapid testing. The most widely used tests today (i.e., RT-PCR) are slow, expensive, and require highly skilled workers and designated lab space to collect and interpret the results.
Solution:
This SARS-CoV-2 biosensor is fabricated on cotton swabs and can be manufactured at low cost. This technology is extremely portable without the need of any heavy equipment for SARS-CoV-2 detection, enabling use at the point-of-care and at home. It also allows SARS-CoV-2 detection within 5 minutes in two simple steps, and the test result can be assessed with naked eye while quantitative results can also be generated using a smartphone camera and a free app.
Technology:
The COvid-19 Low Cost Optodiagnostic for Rapid Testing (COLOR) consists of a cotton swab whose surface is modified with angiotensin converting enzyme 2 (ACE2), the binding receptor for the spike proteins on SARS-CoV-2. To perform the test, the cotton swabs are immersed into nasopharyngeal or oropharyngeal samples for one minute, then are soaked in a wine-colored AuNP-cys-ACE2 solution for three minutes. If the sample contains SARS-CoV-2, the interactions between the spike proteins, ACE2 on the cotton swabs, and the gold nanoparticles (AuNP) induce aggregation of AuNP, which subsequently cause the color shift from wine to purple. Detection can occur qualitatively with naked eye or quantitatively through a smartphone camera and a free app.
Advantages:
- Can be manufactured for $0.15 with readily available materials
- Allows detection of SARS-CoV-2 within 5 minutes and without any lab equipment
- Can be used at home to detect SARS-CoV-2
- Displayed 90% accuracy, 96% sensitivity, and 84% selectivity in a clinical sample study
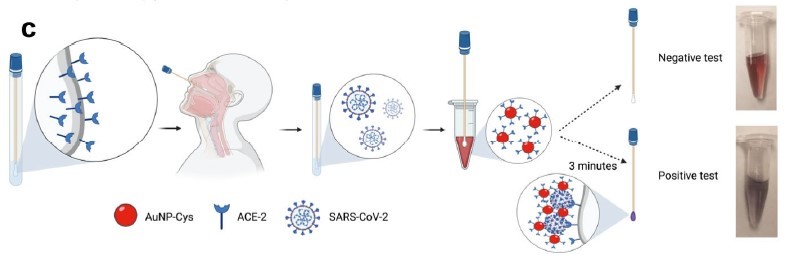
Schematic of COLOR technology in SARS-CoV-2 diagnosis: 1) ACE2 immobilization on the surface of cotton swab, 2) sample collection, 3) attachment of SARS-CoV-2 to the swab, 4) incubation in AuNP-cys-ACE2 solution, and 5) color changes of both the swab and the AuNP-containing solution due to AuNP aggregation in the presence of SARS-CoV-2 (bottom) or no color change without the presence of SARS-CoV-2 (top).
Case ID:
21-9678-TpNCS
Web Published:
6/26/2024
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |