Problem:
Hereditary tyrosinemia type 1 (HT1) is a genetic metabolic disease caused by the inability to break down tyrosine, an amino acid present in many meats, dairy products, fruits, and nuts. This disease can be fatal within the first few months of life and increases the risk of liver cancer. The current treatment involves a strict drug regimen and dietary restrictions. Many people are unable to maintain this regimen and require a liver transplant. Therefore, patients need a lasting cure for HT1.
Solution:
Inventors developed a gene-editing method to restore tyrosine processing and cure HT1. The cure can be administered to patients with HT1 or to a fetus before birth. This method targets a non-mutated gene, making it universal for all patients regardless of their specific causal mutation. Additionally, the gene-editing method is highly specific to the target gene and the liver, minimizing off-target effects.
Technology:
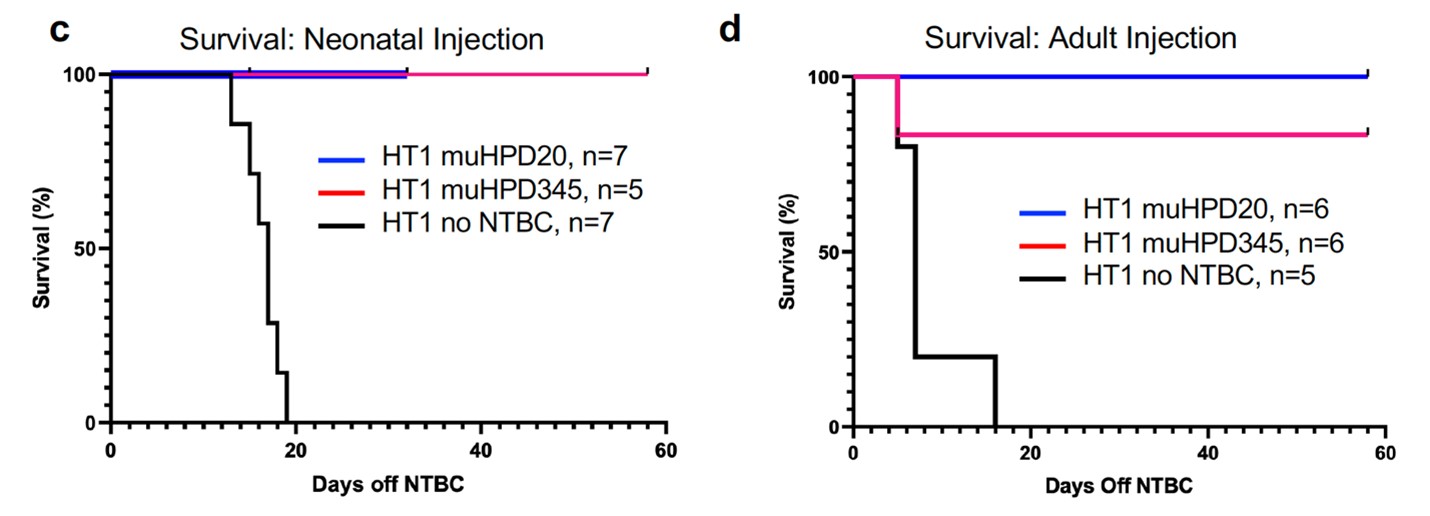
The inventors developed a gene-editing strategy to inhibit the function of 4-Hydroxyphenylpyruvate dioxygenase (HPD) that acts upstream of the HT1-mutated enzyme (FAH). This loss of HPD function shunts the tyrosine byproducts to an alternative pathway. Specifically, the inventors used an adenine-base editor and guide RNA that targets a splice site in HPD. They packaged the editor and guide RNA in lipid nanoparticles to enhance delivery to the liver. These lipid nanoparticles can be injected by a one-time IV infusion.
Advantages:
- One-time durable cure eliminates the need for lifelong drug treatment and dietary restrictions.
- Lipid-nanoparticle delivery increases on-target delivery to the liver (60-70% of alleles edited) rather than off-target organs (0-1.5% edited).
- Adenine base editing of a splice site has high on-target efficiency and low off-target editing: 60-70% of target alleles were edited, while none of the top 100 predicted off-target sites were edited.
Stage of Development:
- Target Identified
- Preclinical Discovery
- IND Enabling Studies
Intellectual Property:
- US & Canadian Patents Filed
Case ID:
23-10295-TpNCS
Web Published:
7/10/2024
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |