Using mRNA-LNPs to deliver forkhead box protein 3 (Foxp3) to CD4+ T cells to engineer Foxp3-positive T (FP3T) cells with immunosuppressive properties.
Problem:
Autoimmune disorders (e.g., type 1 diabetes, multiple sclerosis, and myasthenia gravis) are mediated by self-reactive lymphocyte (B or T cells) expansion. Available suppressive medications like steroids, require frequent dosing and are non-specific, resulting in systemic immunosuppression and increased susceptibility to infections. Adoptive cell therapy is a safer and targeted alternative as it utilizes patient-derived cells to achieve immunosuppression and limits extreme side effects such as cytokine release syndrome. Specifically, regulatory T cells (Tregs) have gained attention due to their critical role in immunosuppressive modulation. However, harvesting, purifying, and growing Tregs to therapeutically relevant numbers is challenging.
Solution:
The inventors identified a proprietary ionizable lipid (C14-A1) and subsequent lipid nanoparticle formulation and encapsulated Foxp3 mRNA in the LNP to target and convert primary human CD4+ T cells into Foxp3-positive T (FP3T) cells that function like immunosuppressive Tregs ex-vivo. The FP3T cells have a cytokine profile and suppressive functionality like endogenous Tregs.
Technology:
From a library of ionizable lipids (an essential component of LNP) including both polyamine cores containing ether linkers (E1-E3) and their analogous structures containing alkyl linkers (A1-A3), the inventors identified C14-A1 as the top-performing lipid with high transfection efficiency and low toxicity. Using microfluidic mixing to formulate LNPs that encapsulate mRNA encoding Foxp3 (a master transcription factor that causes T cells to suppress unwanted effector CD4+/CD8+ cell proliferation by upregulating suppressive cytokine (IL-10 and TGF-β1) secretion), the team delivered the Foxp3 mRNA-LNP formulation to primary human CD4+ T cells to induce Treg-like cells.
Advantages:
- Newly identified ionizable lipid, C14-A1, shows improved mRNA delivery to T cells compared to industry standards, like C12-200.
- Safe and potent ex-vivo engineering of primary human T cells as immunotherapies for autoimmune disorders (e.g., type 1 diabetes, myasthenia gravis, rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis).
- Facilitated greater intracellular mRNA expression, indicated by significant (p< 0.01) 2-3 fold increase in median fluorescence intensity (MFI).
- Potential application in graft-versus-host disease prevention after organ transplantation surgeries.
- Potential application in hypersensitivity during allergic reactions.
- Potential non-viral and in-vivo gene (mRNA) delivery to T cells.
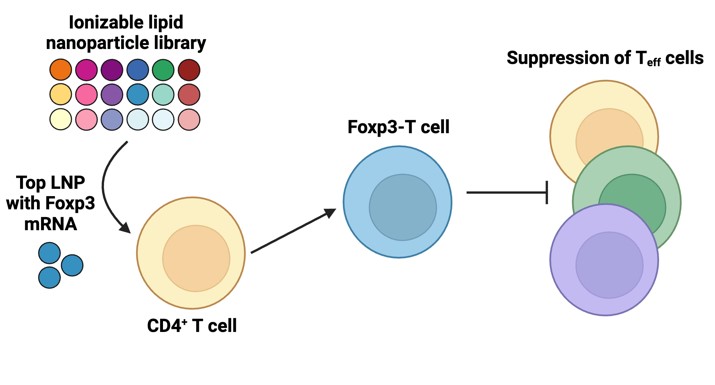
Figure 1: Graphical Abstract: Development of a mRNA lipid nanoparticle (mRNA-LNP) platform for immunosuppressive Foxp3-T (FP3T) cell engineering. (A) Schematic of Foxp3 mRNA-LNPs delivered to CD4+ T cells to generate Foxp3-T (FP3T) cells that can suppress effector T cell proliferation. (B) Chemical structures of six polyamine cores with either alkyl (A1–A3) or ether (E1–E3) spacers. (B) Chemical structures of epoxide tails with three varying carbon chain lengths─C12, C14, or C16. (C) Scheme of the SN2 reaction chemistry used to synthesize each unique ionizable lipid.
Case ID:
23-10394-TpNCS
Web Published:
10/1/2024
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |