Using Mannich reaction-based combinatorial libraries to identify ionizable lipids for low-immunogenic and high-efficiency mRNA delivery.
Problem:
Lipid nanoparticle (LNP)-based delivery systems for mRNA-based therapeutics have recently demonstrated their clinical success in applications such as ONPATTRO® and the Pfizer/BioNTech and Moderna COVID-19 vaccines. However, existing LNP-based delivery systems have intrinsic high immunogenicity, restricting the mRNA therapeutics' protein expression levels, durability, and broader applications (e.g., redosing).
Solution:
The inventors constructed a series of Mannich reaction-based combinatorial libraries and used high throughput in vitro and in vivo screenings to identify the top-performing ionizable lipid, C-a16. This antioxidant lipid effectively reduces cellular inflammatory responses, extends intracellular protein expression duration, and mitigates intracellular reactive oxygen species (ROS), a pro-inflammatory signal induced by LNP-mRNA treatment.
Technology:
The inventors designed six combinatorial libraries utilizing Mannich reactions between (1) amines, (2) formaldehyde, and (3) various compounds such as phenols, thiols, dialkyl ketones, pyrroles, and benzophenones. This approach established a comprehensive screen comprising structurally diverse ionizable lipids (310 in vitro and 11 in vivo). They identified the strongest candidate, C-a16 lipid, and compared its expression level, half-life, and immunogenicity with different commercially available ionizable lipids (e.g., MC-3 and SM-102). Then, the team performed further proof-of-concept studies to demonstrate the superiority of the C-a16 LNP in delivering mRNA therapeutics encoding gene editor, tumor neoantigen, and SARS-CoV-2 spike protein.
Advantages:
Use Mannich reactions to establish combinatorial ionizable lipid libraries with higher structure diversity and physicochemical properties.
A newly identified ionizable lipid, C-a16, shows improved mRNA delivery and lower immunogenicity than commercially available lipids, such as MC-3, SM-102, and ALC-0315.
- ~5-fold higher luminescence expression intensity compared to MC-3 LNP.
- ~30% increase in the half-life of luciferase in C-a16 LNP-treated mice (53 hours) compared to MC-3 LNP-treated mice (40 hours).
- C-a16 LNPs contain phenolic tails, which display significantly greater transfection efficiency than other lipids with similar carbon numbers on the tails.
- Significantly lower expression of oxidative stress-, cell death-, innate immune activation-, RNA- and protein-degradation genes in C-a16 LNP-treated cells.
- Significantly Lower reactive oxygen species (ROS) in C-a16 LNP-treated Mice than in the MC-3 LNP-treated group.
- C-a16 LNPs can effectively traffic to the lymph nodes after intramuscular injection.
- C-a16 LNPs co-delivering Cas9 mRNA and a guide RNA for transthyretin (TTR) genome editing achieved 5-fold higher TTR gene editing than MC-3 LNP.
- C-a16 LNPs delivering fibroblast growth factor 21 (FGF21) mRNA led to a 3-fold increase in FGF21 expression in vivo compared to MC-3 LNP.
- C-a16 LNPs encapsulating tumor neoantigen or SARS-CoV-2 spike protein mRNA induced superior antigen-specific immune responses compared to SM102 LNP.
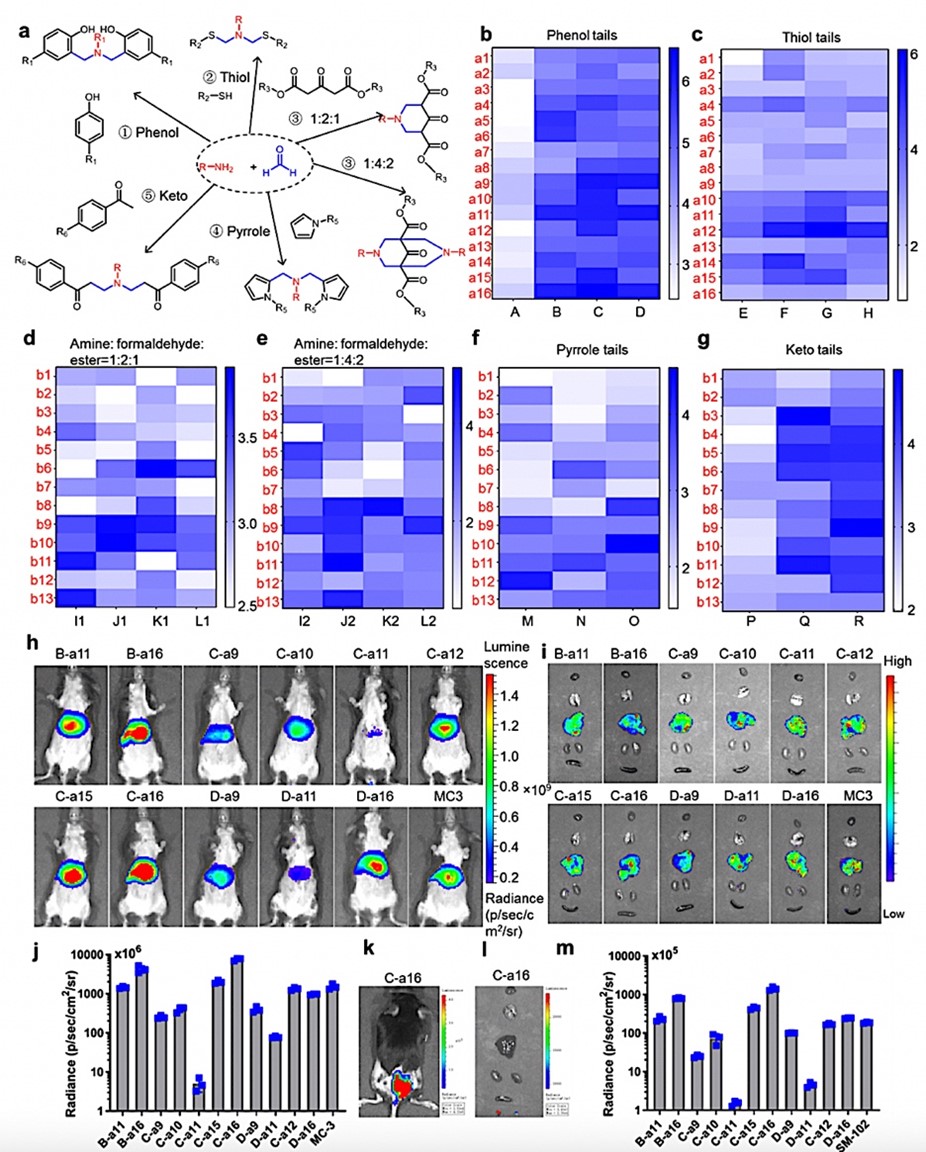
Construction of Mannich reaction-based combinatorial libraries for in vitro and in vivo screening of ionizable lipids. a. Schematic representations of Mannich reactions involving phenol, thiol, dialkyl ketones, pyrroles, and benzophenones for synthesizing structurally diverse ionizable lipids. For different Mannich reactions, the team mixed different derivatives (phenol, thiol, dialkyl ketones, pyrroles, and benzophenones) with formaldehyde and amines at particular molar ratios in ethanol and/or water. b-g. Heat maps illustrating the luminescence levels of MC38 cells (a mouse melanoma cell line) following treatment with various lipid nanoparticles (LNPs). Notably, lipids synthesized using the phenol Mannich reaction (Fig. b) exhibited higher luminescence levels than lipids derived from other Mannich reactions. h-i. Intravenously inject the seven top-performing LNPs (B-a11, B-a16, C-a9, C-a10, C-a11, C-a12, C-a15, C-a16, D-a9, D-a11, and D-a16) in mice and examine the biodistribution of LNPs (Fig. h) and the expression of luciferase in vivo (Fig. i). The results showed that most luciferase signals localize in the liver for all LNPs, with C-a16 LNP displaying the highest expression level. j. Intravenously administered eleven top-performing LNPs in mice and quantified the overall luminescence levels. Mice receiving C-a16 LNPs have approximately five times greater luminescence levels than those with MC-3 LNPs (n=3). k-l. In vivo luminescence signal visualization in mice and organs through IVIS images after intramuscular injection of C-a16 LNPs. m. Quantifying the luminescence levels in mice following intramuscular injection of the eleven top-performing LNPs (n=3). C-a16 LNPs induced the highest luciferase expression at the injection site (Fig. m) and in the lymph nodes (results not shown).