Diverse difluoromethylene scaffolds via novel, organodifluorine synthons.
Problem:
Organofluorine compounds are used in numerous applications across society, including in refrigerants, pharmaceutical ingredients, and agrochemicals. Most current methods to incorporate fluorine atoms into organic compounds use monovalent synthons, which only permit linear syntheses that do not provide a point of diversification.
Solution:
The technology is an ambiphilic organodifluoromethylene synthon that can be used in a one-pot three-component reaction to create a variety of adducts with high diasteroselectivity.
Technology:
This technology is a one-pot three-component union with Anion Relay Chemistry (ARC) by using an ambiphilic linchpin in which the anionic carbon is stabilized by geminal fluorine substituents. α, β- unsaturated aldehyde electrophiles and enantioenriched sulfonyl imine electrophiles are amenable to the reaction. Alkyl, vinyl, alkynyl, aryl, and dithianyllithium reagents are viable nucleophiles in the reaction as well.
Advantages:
- One-pot synthesis
- Divergent strategy
- Three-component adduct—a variety of nucleophiles, linchpins, and electrophiles can be employed
- Functional group tolerance
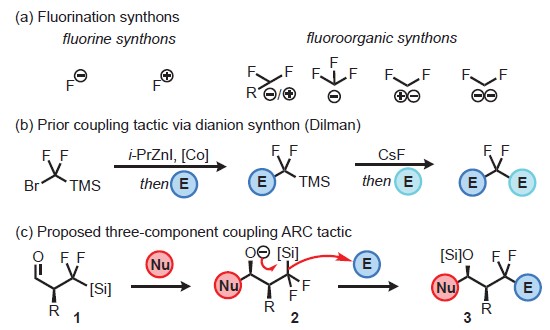
(a) Fluorination synthons; (b) Prior coupling tactic employing a dianion synthon; (c) fluoroorganic synthon and three-component coupling tactic via Type II Anion Relay Chemistry (ARC).
Case ID:
21-9554-TpNCS
Web Published:
10/3/2024
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |