The base editing of phenylalanine hydroxylase enzyme in the liver to restore phenylalanine metabolism and prevent neurotoxic effects.
Problem:
Phenylketonuria (PKU) is a severe disorder affecting approximately 1 in 10,000 U.S. newborns. PKU is marked by a genetic mutation in the phenylalanine hydroxylase enzyme (PAH), resulting in phenylalanine (Phe) buildup. Early detection and management through strict dietary Phe restriction can prevent severe intellectual disability. Additional treatments include large neutral amino acids and glycomacropeptide supplementation, though with limited success. Finally, there are the FDA-approved adjuvant treatment for PKU, sapropterin dihydrochloride, and the enzyme substitution therapy pegvaliase, which also have limited efficacy. Owing to the restricted therapeutic modalities for PKU, novel approaches are required to fill this unmet need.
Solution:
This invention offers a genetic engineering approach to restore PAH function through the base editing of mutant PAH that enables sustained production of functional PAH capable of restoring Phe metabolism.
Technology:
This invention corrects one of the most common PAH variants in PKU (P281L) through corrective adenine base editing (ABE) of mutated PAH. Although the primary toxicity is in the brain, the PAH gene is largely expressed in the liver from hepatocytes presenting them as the primary therapeutic target. With the delivery of ABE8.8 mRNA and the P281L guide RNAs in vivo using lipid nanoparticles (LNPs) in humanized PKU mice, a complete and durable normalization of blood Phe levels occurs within 48 h of treatment, resulting from corrective PAH editing in the liver.
Advantages:
- First-in-class curative strategy for PKU.
- First genetic engineering approach in PAH.
- LNPs can be used to deliver base editors in vivo, which naturally hone to the liver in vivo.
- Alternative strategies include AAV delivery.
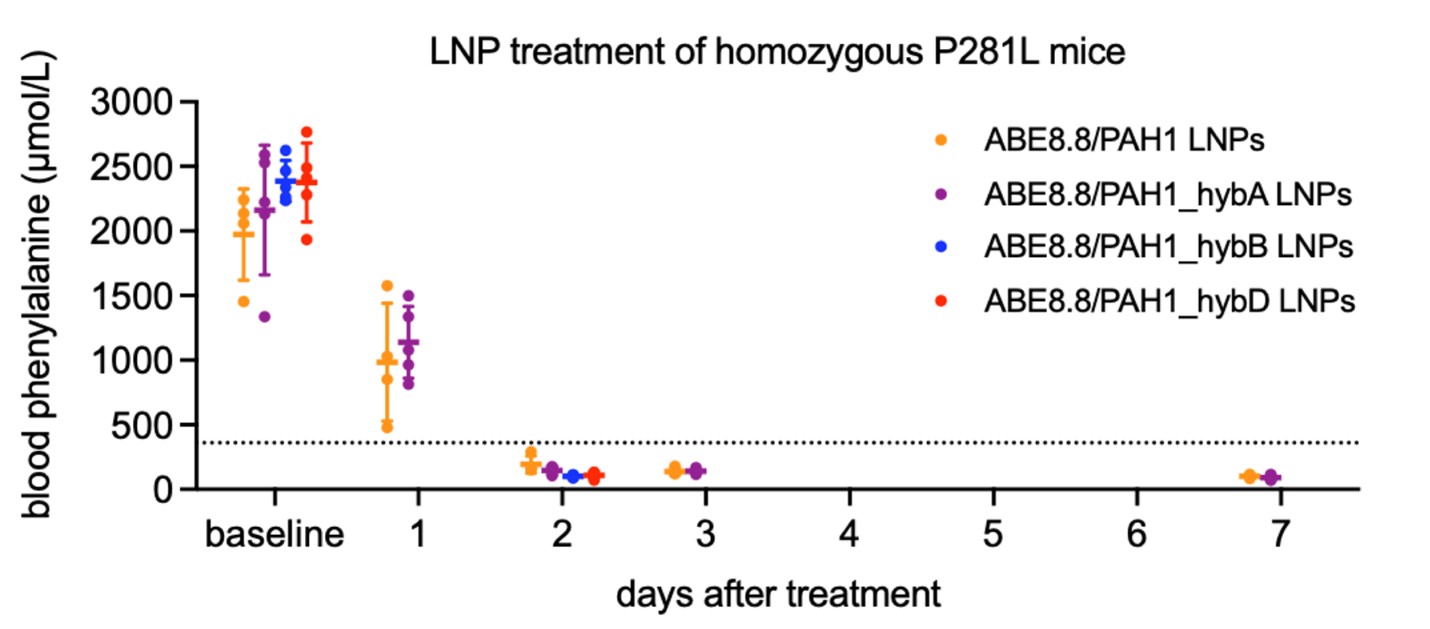
Improved on-target editing in humanized PKU mice with PAH1 hybrid guide RNAs. Blood phenylalanine levels in mice reduce over time, below the recommended threshold (<360 μmol/L) for PKU patients after treatment with a 2.5 mg/kg dose of LNP base editors in both the normal and optimized hybrid (hyb) guides iterations, which have higher levels of editing specificity
Case ID:
22-10020-TpNCS
Web Published:
10/28/2024
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |