CAR-T cells are PEGylated to block the interactions with monocytes and macrophages to reduce the side effects of CAR-T cell therapies (cytokine release syndrome and neurotoxicity).
Problem:
CAR-T cell therapies lead to many adverse side effects such as cytokine release syndrome (CRS) and neurotoxicity. It has been shown that 20-70% of patients receiving CD19 CAR-T cell therapy will develop CRS. This gives rise to many symptoms such as high fever, increased levels of acute-phase proteins, and respiratory and cardiovascular insufficiency, and if left untreated, could lead to multiple organ dysfunction or patient death.
Solution:
A method involving PEGylation of CAR T cells to alleviate side effects of CAR T cell immunotherapies. Polyethylene glycol (PEG) acts as a biodegradable polymeric spacer to block cell-to-cell interactions. PEG600k, MW 600k has PEG length of about 6 μm, which is much longer than the other PEGs tested and may contribute to greater steric hindrance. This helps block the cell-to-cell interactions between the CAR T cells and monocytes and macrophages in order to prevent CRS and neurotoxicity.
Technology:
Dibenzocycloctyne (DBCO)-PEG600k is conjugated to CAR T cells via azide-alkyne click chemistry. DBCO-PEG600k acts as a spacer molecule to block interactions between CAR-T cells and the monocytes and macrophages. In reponse, PEG-modified CAR-T cells and monocytes expand slowly. This slower expansion allows for PEG spacer to be diluted, which provides a window for CAR-T cells to kill tumour cells before CAR-T cell induced monocyte over-activation. This method is more effective than systemically administered drugs (i.e. tocilizumab) that are used to reduce CRS and neurotoxicity in the clinic.
Advantages:
- Decrease in both monocyte activation and cytokine release in vitro via modification of CAR-T cells with DBCO-PEG600k.
- DBCO-PEG600k at higher concentrations of 10 and 50 mg/kg greatly reverses weight loss, high fever and cytokine release in mice.
- DBCO-PEG600k protects the mice from severe neurotoxicity and alleviates CRS to a greater extent than that the commonly used treatment of tocilizumab.
- DBCO-PEG600k significantly prolongs mouse survival, with no signs of neurotoxicity even at Day 37.
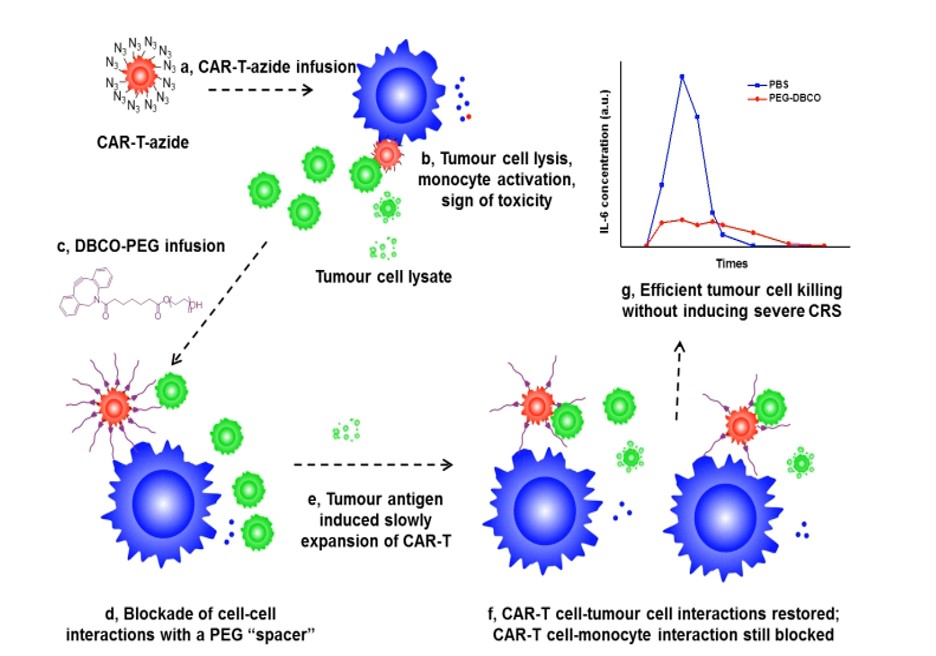
a) CAR-T azide infusion b) Tumor cell lysis, monocyte activation, sign of toxicity (c) DBCO-PEG infusion (d) Blockade of cell-cell interactions with a PEG “spacer” (e) Tumor antigen induced slowly expansion of CAR-T (f) CAR-T cell-tumor cell interactions restored, CAR-T cell-monocyte interaction still blocked (g) Efficient tumor cell killing without inducing severe CRS.
Stage of Development:
- Target Identified
- Preclinical Discovery
Case ID:
22-10066-TpNCS
Web Published:
12/20/2024
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |