A method of combining T2R agonists and GLUT1 inhibitors to treat head and neck cancers.
Problem:
Head and neck squamous cell carcinoma (HNSCC) is a significant health concern with a high incidence and mortality rate. Approximately half of patients succumb to disease within 5 years. Current treatments for HNSCC include surgery, radiation therapy, chemotherapy, and occasionally immunotherapy; each of these treatments is associated with substantial morbidity. Despite these options, the survival rate for advanced HNSCC remains low due to high rates of treatment-resistant recurrent disease.
Solution:
The proposed solution involves using bitter taste receptor (T2R) agonists, such as T2R14 agonist lidocaine, to selectively induce apoptosis in cancer cells. This approach can be combined with other therapeutics, such as GLUT1 inhibitors, to enhance efficacy and reduce toxicity.
Technology:
The inventors approached the problem by identifying T2R14 as a novel target and biomarker for HNSCC. They discovered that activation of T2R14 by lidocaine triggers apoptosis in HNSCC cells, particularly HPV associated tumors which are enriched for this receptor. Their lab explored the potential of combining these agonists with other drugs including GLUT1 inhibitors, demonstrating improved cytotoxicity and potential to improve treatment outcomes. This innovative approach leverages the unique properties of T2R14 to selectively target cancer cells while sparing normal tissues with lower expression of this receptor.
Advantages:
- Selective targeting of cancer cells
- Potential for combination therapy to enhance efficacy and reduce toxicity
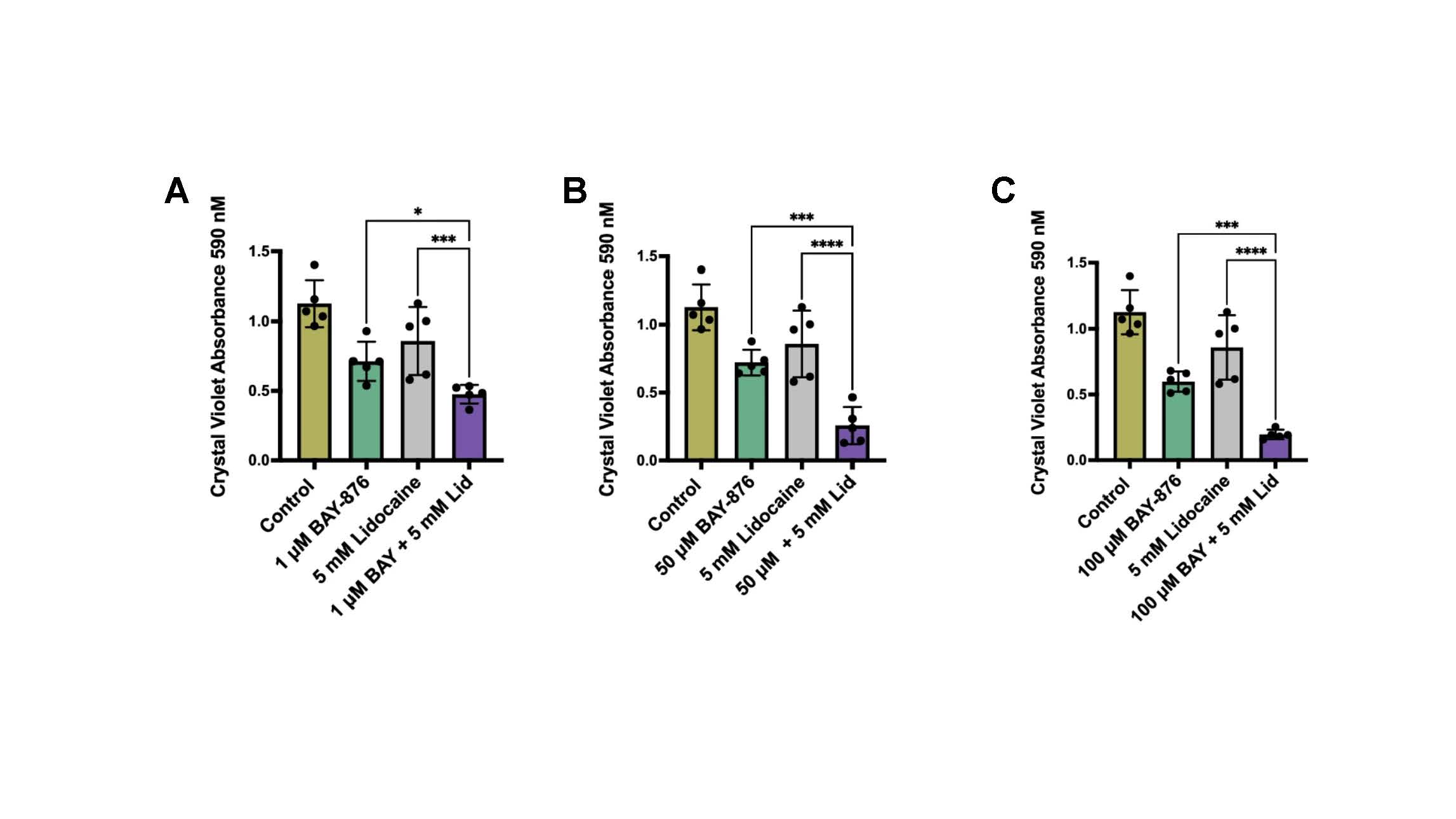
Crystal violet absorbance representing live/adherent SCC47 cells following treatment with 1–100 μM BAY-876± 5mM lidocaine (A-C).
Stage of Development:
- Target Identified
- Preclinical Discovery
- Phase 1 RCT in humans exploring feasibility and efficacy of T2R14 lidocaine monotherapy in HNSCC (NCT06747390)
Case ID:
24-10707-TpNCS
Web Published:
5/21/2025
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |