Synergistic combination of mechanoporation and lipid nanoparticle (LNP) based transfection for an efficient, non-viral method of gene transfer to generate CAR-T cell therapies.
Problem:
Chimeric antigen receptor (CAR)-T cell therapy has transformed cancer treatment by engineering patients' T cells to precisely target and eradicate cancer cells. Conventional methods of CAR-T production rely on lentiviral transduction to integrate CAR genes into target cells. However, viral-based transduction can cause adverse effects and provoke unwanted immune responses such as cytokine release syndrome. Non-viral methods of gene transfer are safer but are not as efficient and can produce high cytotoxicity.
Solution:
The inventors achieve efficient and non-lethal gene delivery through LNP + Squeeze, an microfluidic-based intracellular delivery platform that combines mechanoporation and LNP based transfection. The inventors found that inducing temporary cell membrane disruption through mechanoporation permitted successful delivery of LNPs into cells. This combination can work synergistically to generate CAR-T cells.
Technology:
The inventors have created a system in which pDNA (plasmid DNA) is mixed with LNPs in a microfluidic device. This system has been used by the inventors to package CAR plasmids into LNPs. The pDNA-loaded LNPs are subsequently fed into a distinct microfluidic device with multiple gaps, which “squeeze” the cells and enable pDNA-loaded LNP delivery to the cells via the generation of transient pores on the cell membrane (mechanoporation). The squeeze regions are highly parallelized with 20 gaps per row and multiple rows assembled into a compact microfluidic device. The inventors have used this system to physically deliver CAR pDNA-loaded LNPs into Jurkat cells and primary ɑβ T cells to generate CAR-expressing cells. The combination of mechanoporation and LNP-based transfection improves the efficiency of payload delivery compared to either technique alone.
Advantages:
- Design of parallelized microfluidic device (gaps instead of channels) avoids cell clogging
- Low cytotoxicity and high expression of delivered cargo, such as pDNA
- Efficient generation of CAR-T cell products
- Transfection efficiency nearly doubled compared to electroporation or LNP transfection alone in Jurkat cells and primary ɑβ T cells
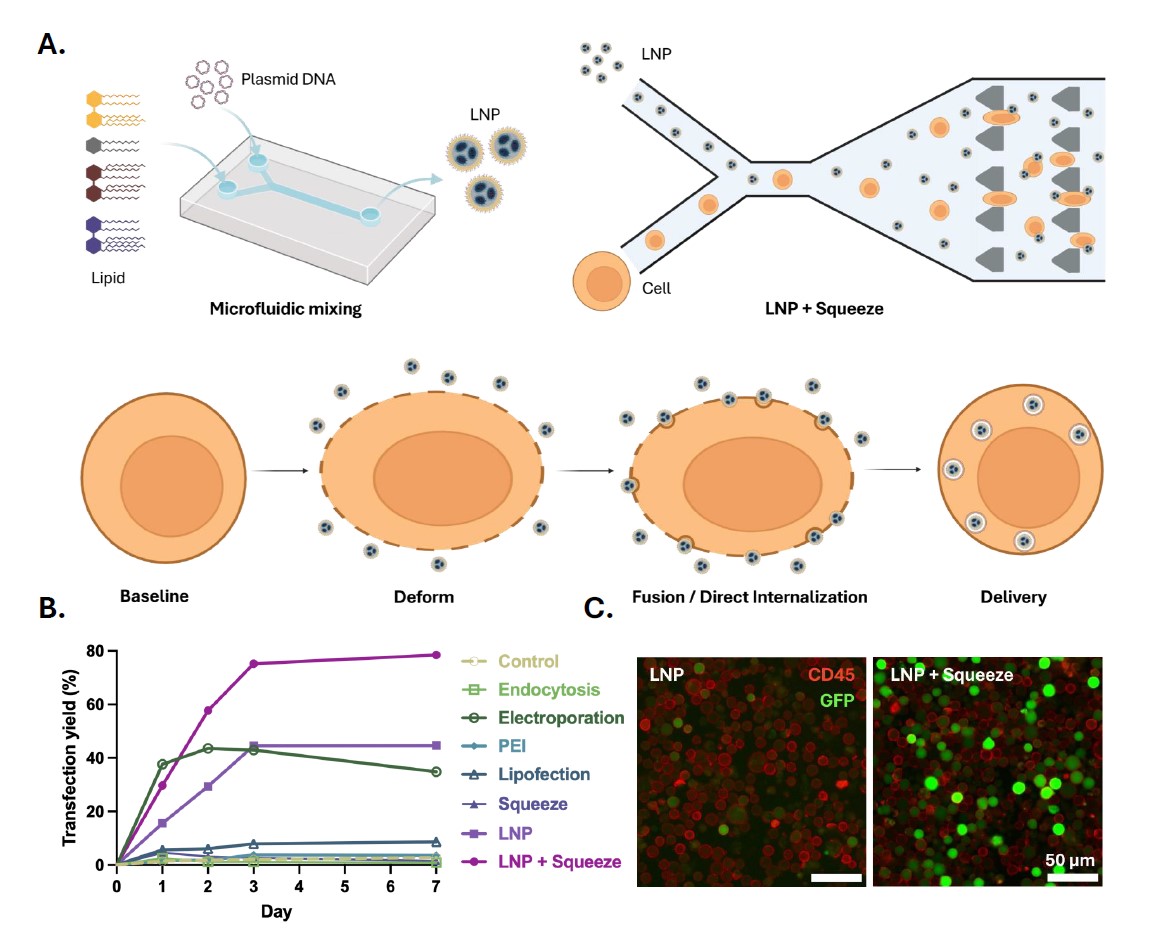
Figure A is a cartoon rendering of the microfluidic device used for LNP synthesis as well as the Squeeze microfluidic design which combines LNPs and cells. A cartoon depiction of how LNP + Squeeze utilizes mechanoporation to permit cellular uptake is shown at the bottom of Figure A. Figure B includes results from transfection yields, (measured by the percentage of Jurkat cells with positive green fluorescent protein (GFP) signal), across various transfection methods over one week. Figure C includes fluorescent images of CD45+ Jurkat cells (red) after transfection with GFP pDNA-loaded LNPs (green). On the right are the results from the LNP + Squeeze method versus LNP-only methods (left). LNP + Squeeze achieved higher transfection yield compared to other methods as shown quantitatively in Figure B and qualitatively in Figure C.
Stage of Development:
- Preclinical Discovery
- Bench Prototype
Case ID:
25-10960-TpNCS
Web Published:
8/28/2025
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |