Simple, plug-and-play biodegradable lipids that form highly potent lipid nanoparticles (LNPs) for CRISPR mRNA delivery, outperforming benchmark gene-editing systems.
Problem:
CRISPR therapies can permanently correct disease-causing genes, but today's CRISPR delivery systems are either too risky or not potent enough. Viral vectors raise red flags on immunity, safety, cost, and manufacturing, while current LNPs require very high doses to achieve meaningful editing. This potency gap limits how broadly LNP gene editing can safely be applied across genetic diseases. Furthermore, LNP limitations slow development timelines because companies must repeatedly re-engineer new delivery chemistries for each specific therapy.
Solution:
The inventors have developed a two-component technology: "plug-and-play" combinatorial chemistry approach that builds biodegradable, ionizable lipids in a single, high-yield chemical reaction. From a 500-lipid library, the inventors identified several superior candidates, including 5D6.2, 5D8 and 8D6.2. The lead 5D8 lipid formulates into LNPs that deliver CRISPR and siRNA with significantly higher editing and/or gene knockdown than gold-standard lipids. Unlike existing LNP therapies, 5D8 LNPs require significantly lower doses. Thus, 5D8 LNPs fit within relevant dosing expectations and exhibit an improved liver safety profile.
Technology:
A solvent-free Michael addition between amines/thiols and dialkyl maleates generates a 500-member library of biodegradable ionizable lipids using accessible building blocks and simple processing. Lead lipid 5D8 forms LNPs that achieve about 61% on-target liver editing of the transthyretin (TTR) gene and reduces TTR levels in serum by roughly 90%, outperforming benchmark C12-200 and other industry lipids. In addition to delivering CRISPR mRNA for gene editing, 5D8 LNPs efficiently deliver base editors and siRNA in vivo with minimal liver toxicity.
Advantages:
- One-step, plug-and-play synthesis (>80% yield) from commercially accessible starting materials; no solvent or catalyst required.
- Validated as a platform for CRISPR nucleases, adenine base editors, and siRNA, enabling a diversified gene-editing pipeline from a single LNP chemistry.
- GMP-compatible formulation using standard helper lipids and microfluidic LNP manufacturing process.
- Higher liver editing (~61%) and target knockdown than approved or clinical ionizable lipids at similar doses.
- Ultra-low dose efficacy (~100% serum positive control reduction with siRNA at 0.05 mg/kg, 50× lower than typical amounts).
- Biodegradable design with a favorable liver safety profile; no elevation of alanine transaminase (ALT)/aspartate aminotransferase (AST), both of which are typical indicators of hepatotoxicity.
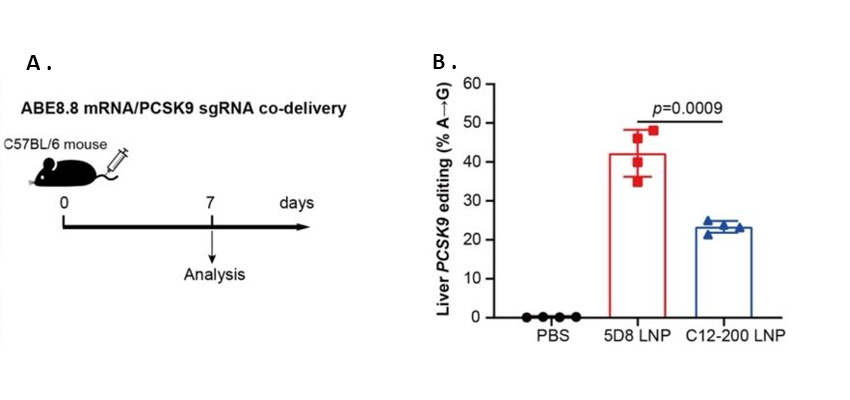
LNP-mediated in vivo gene editing using adenine base editing (ABE). (a,b) LNPs encapsulating ABE8.8 mRNA/PCSK9 sgRNA were i.v. injected into C57BL/6 mice at a total RNA dose of 0.75 mg/kg. On day 7, DNA was extracted from the liver to determine on-target indel frequency by next-generation sequencing, yielding ~40% 5D8 LNP editing efficiency versus ~20% editing efficiency with standard-of-practice C12-200 LNP.
Stage of Development:
- Concept
- Proof of Concept
- Bench Prototype
Case ID:
24-10674-TpNCS
Web Published:
1/14/2026
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |